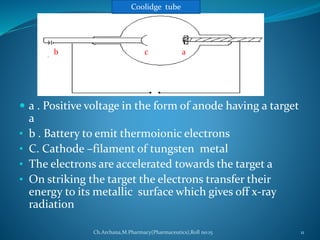
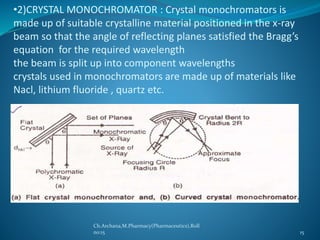
This document provides an overview of X-ray diffraction presented by Archana. It discusses the discovery of X-rays, the generation of X-rays, Bragg's law which describes the diffraction of X-rays by crystals, and the instrumentation used including X-ray sources, monochromators, detectors. It also describes different X-ray diffraction methods such as Laue, Bragg, rotating crystal and powder methods and their applications in determining crystal structures and lattice parameters.