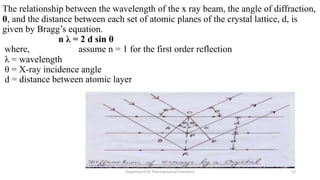
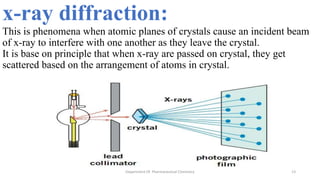
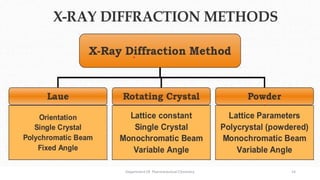
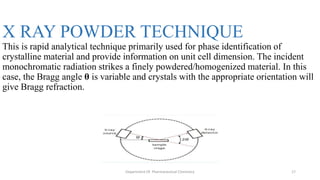
X-ray crystallography is a technique used to determine the atomic and molecular structure of crystals. When X-rays interact with a crystal, the atomic planes cause the X-rays to diffract into specific directions. This diffraction pattern can be used to reveal the crystal structure. Bragg's law describes the angles for diffraction based on the wavelength and spacing of atomic planes. X-ray crystallography techniques include Laue and rotating crystal methods and are used in applications such as determining molecular structures, analyzing protein interactions, and evaluating drug purity and stability.