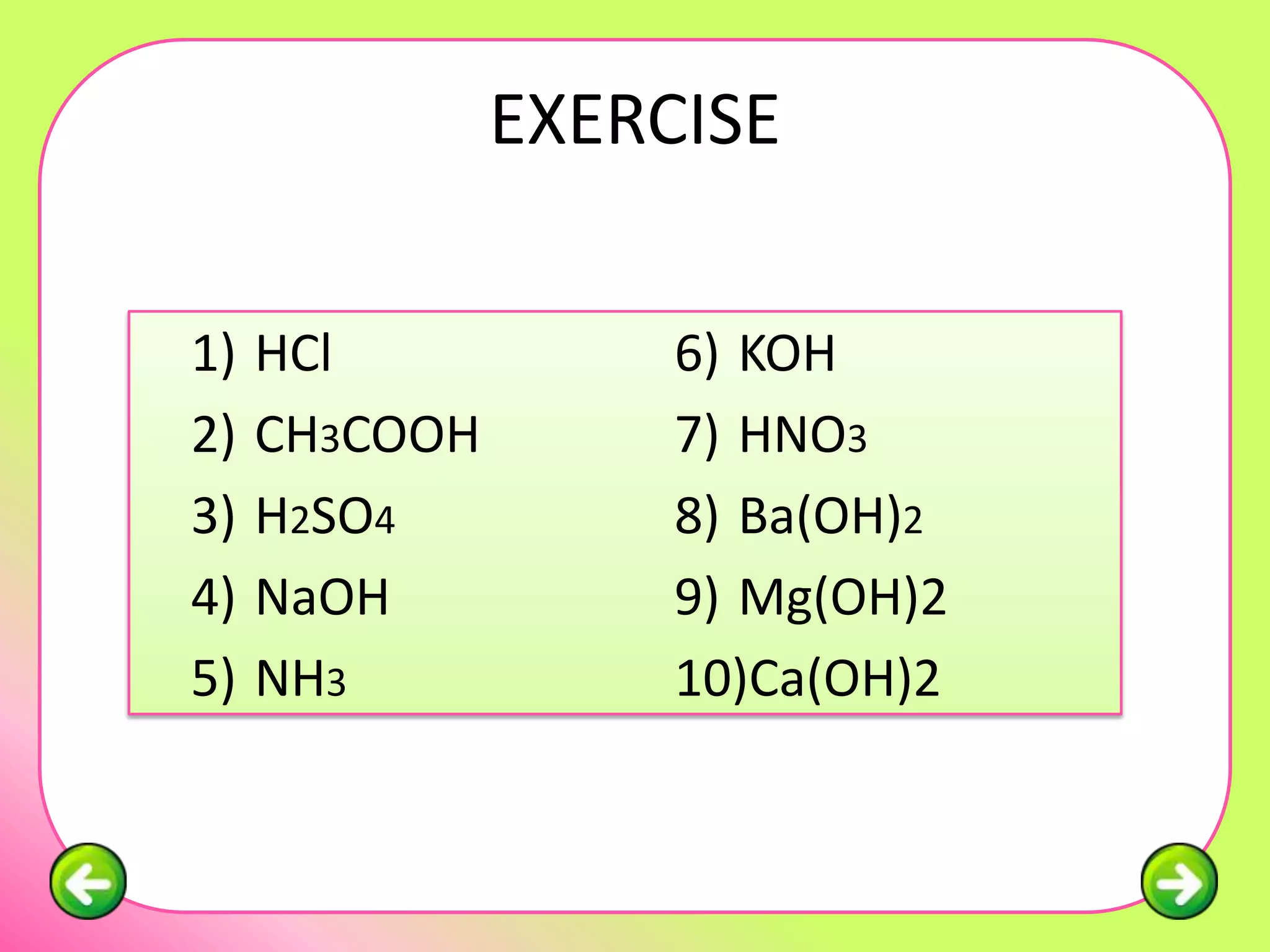
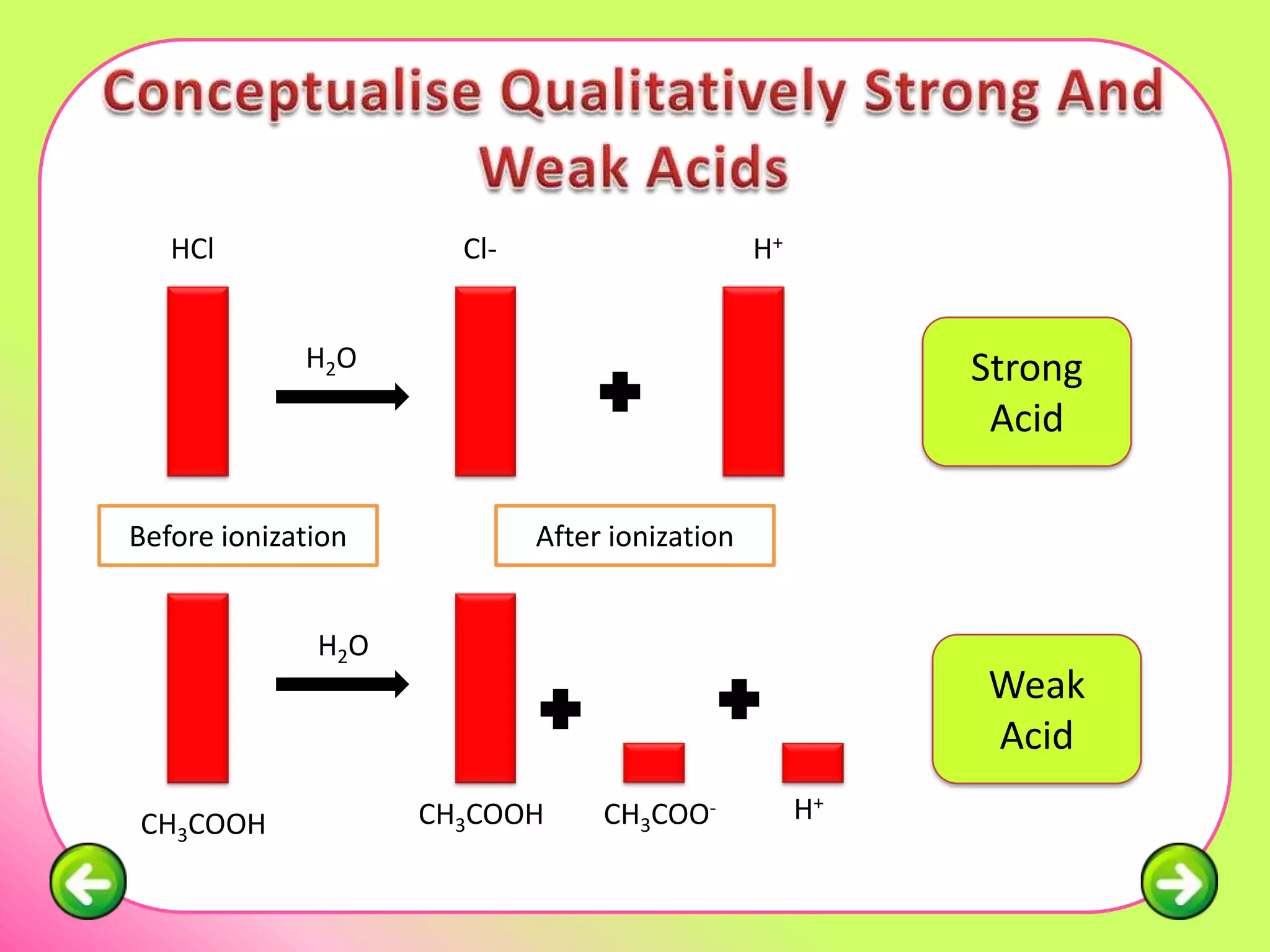
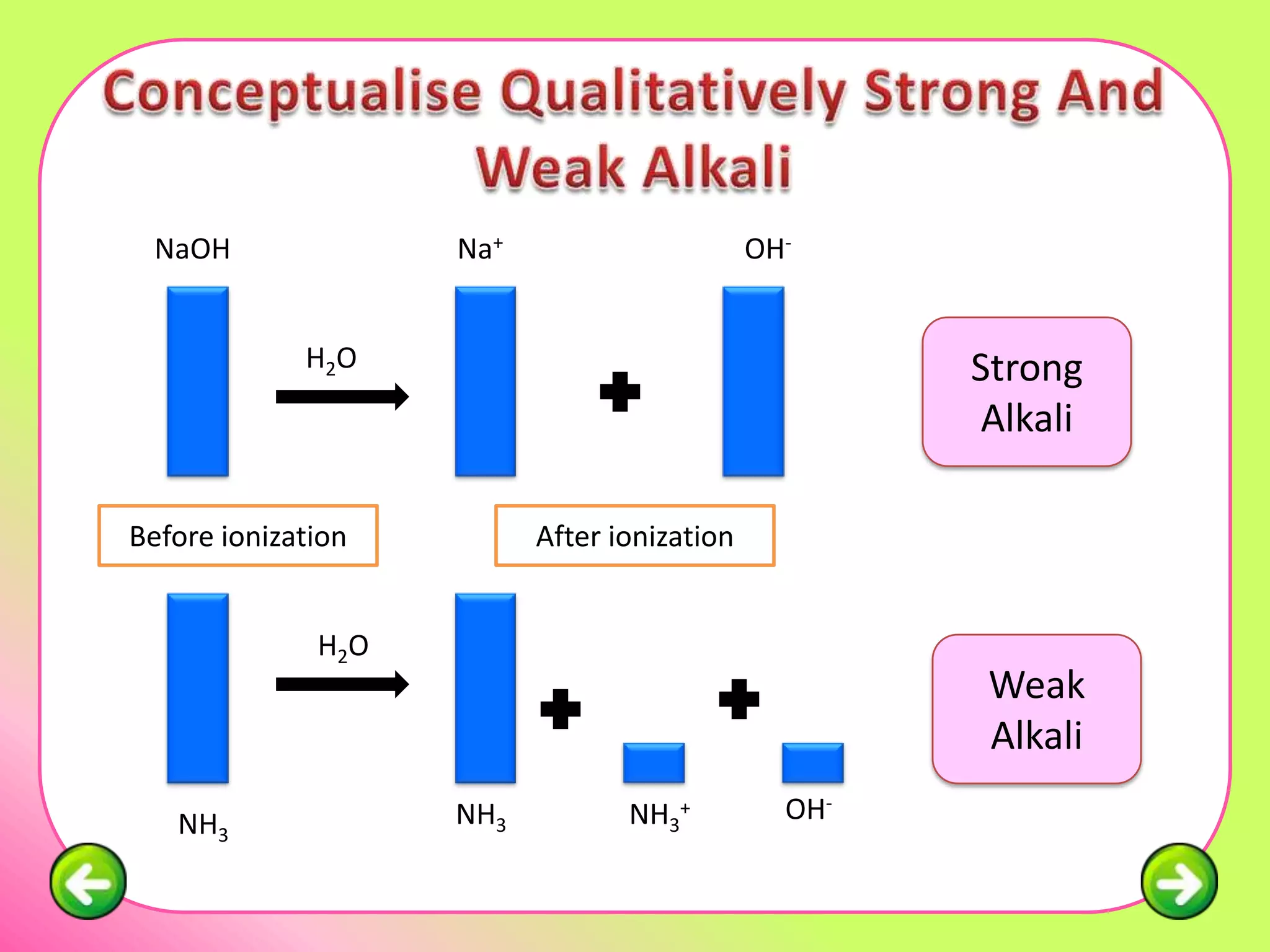
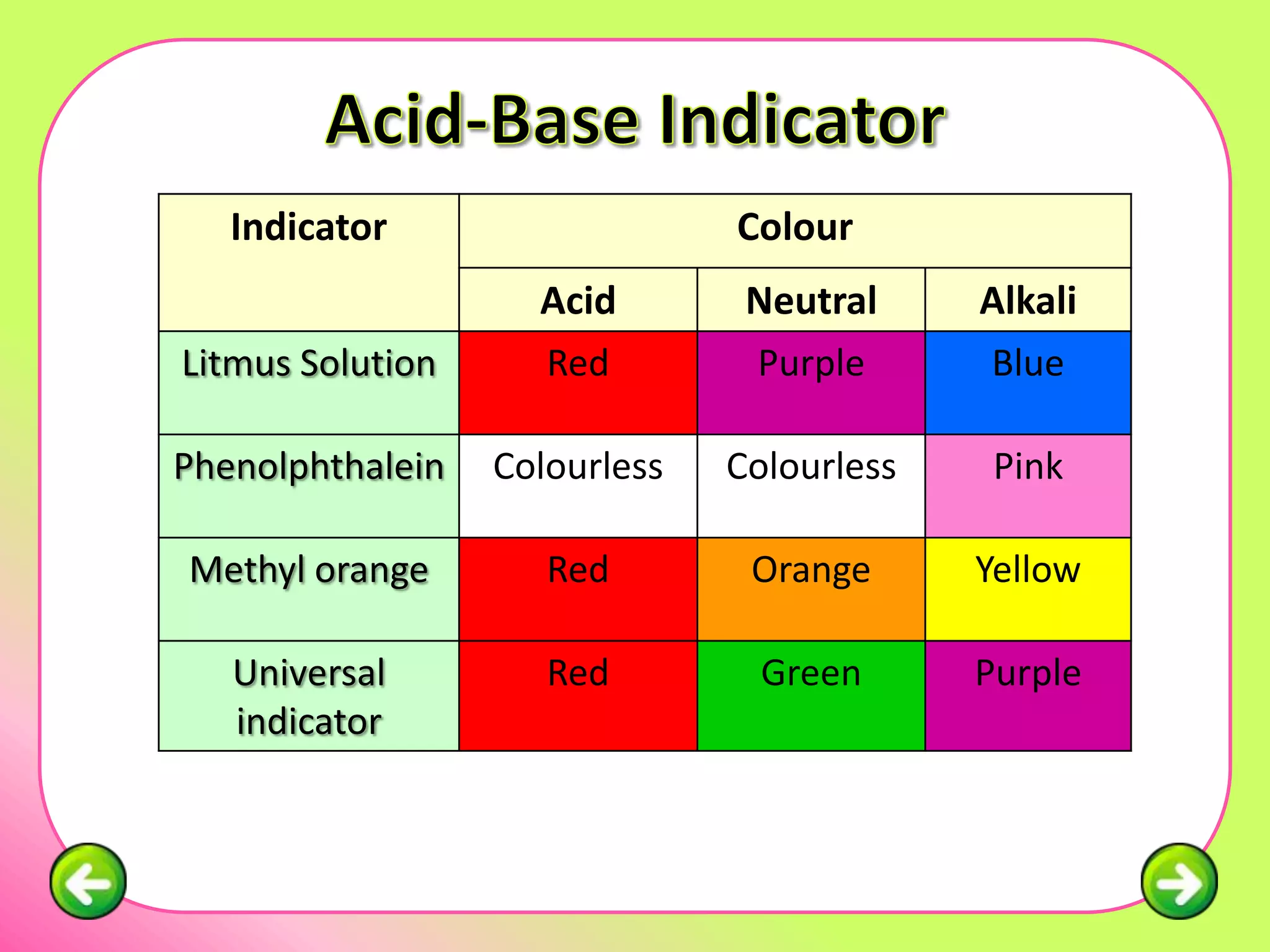
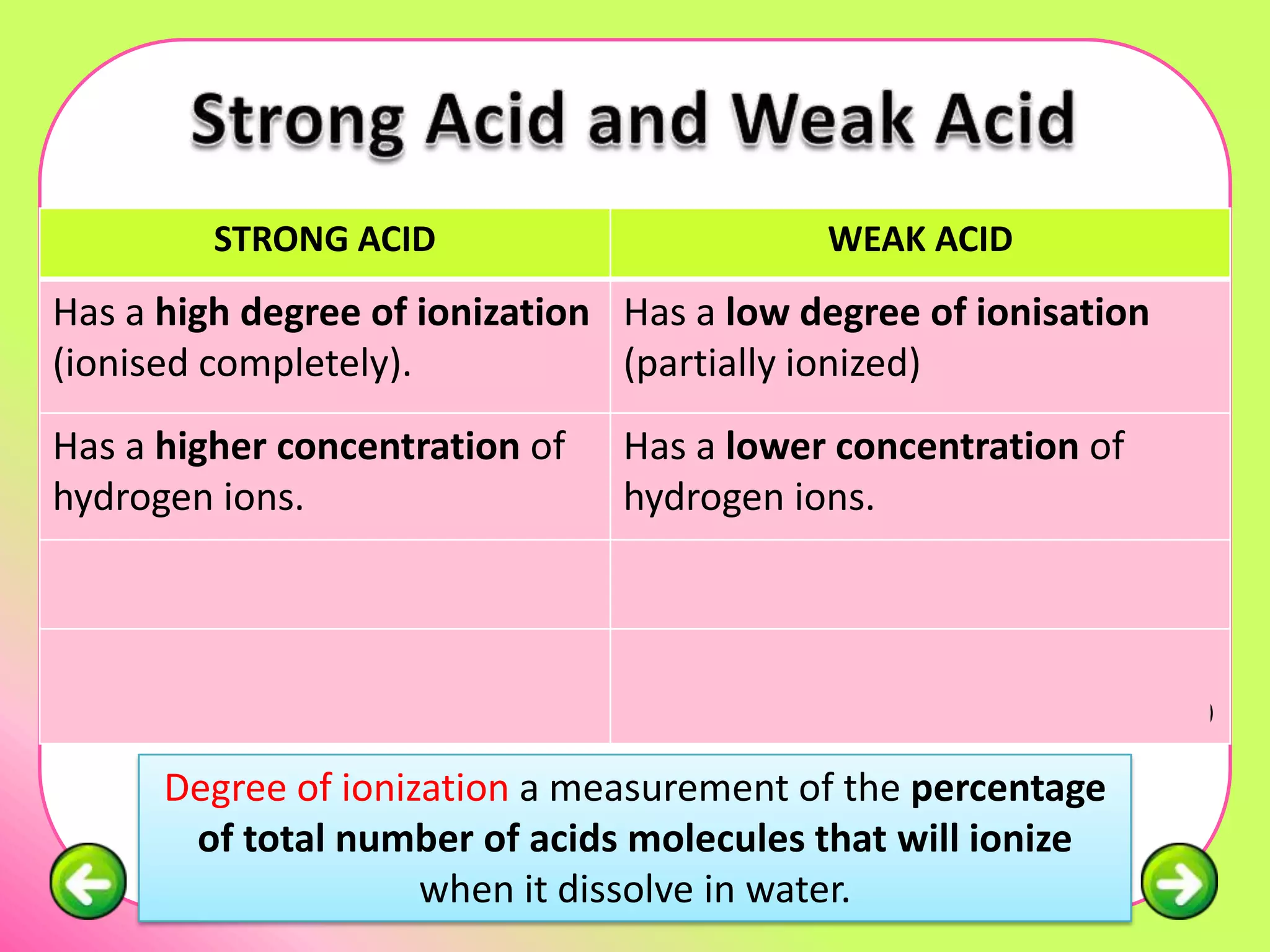
The document discusses pH scales and the properties of acids and bases. It defines pH as a measure of hydrogen ion concentration and relates pH values to whether a substance is acidic or alkaline. It explains that strong acids and bases fully dissociate in water, producing more hydrogen or hydroxide ions and having higher concentrations of these ions compared to weak acids and bases that only partially dissociate. Common indicators are described that change color depending on whether a solution is acidic, alkaline or neutral. Examples of strong versus weak acids and bases are provided.



![To indicate the degree of acidity or alkalinity of asolution
[H+] increases [OH-] increases
Increasing acidity neutral Increasing alkalinity
pH scale 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Weak Alkali Strong Alkali
Strong Acid Weak Acid
Example: NH3 Example:
Example: HCl, Example:
NaOH, KOH
HNO3, H2SO4 CH3COOH,
H2SO3, H3PO4
pH [H+] [OH-]](https://image.slidesharecdn.com/chapter7acidbasespart2-130226001655-phpapp01/75/Chapter-7-Acid-Bases-part-2-5-2048.jpg)
![STRONG ALKALI WEAK ALKALI
Has a high degree of ionization Has a low degree of ionisation
(ionised completely). (partially ionized)
Has a higher [OH-]. Has a lower [OH-]
Has a higher pH value. Has a lower pH value.
H2O H2O
Ex: NaOH (l) Na+(aq) + OH- (aq) Ex: Mg(OH)2 (aq) Mg2+ (aq) + OH- (aq)](https://image.slidesharecdn.com/chapter7acidbasespart2-130226001655-phpapp01/75/Chapter-7-Acid-Bases-part-2-7-2048.jpg)