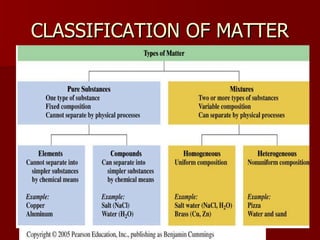
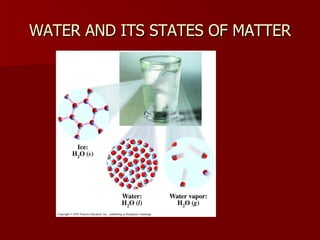
The document introduces chemistry as the study of matter and its transformations, distinguishing between science and technology, with examples of each. It explains the scientific method, outlines the classification of matter (elements, compounds), and reviews physical and chemical changes and properties. Additionally, it provides examples of chemical reactions and outlines how to identify them in practice problems.