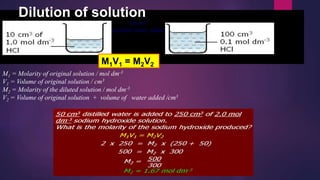
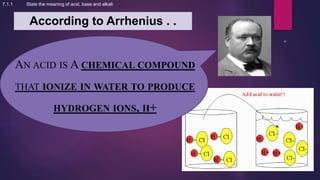
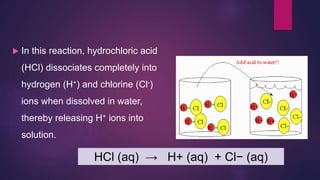
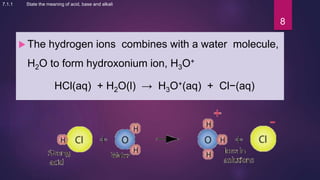
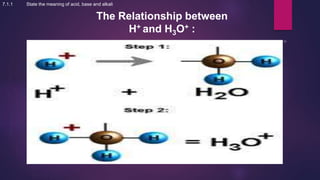
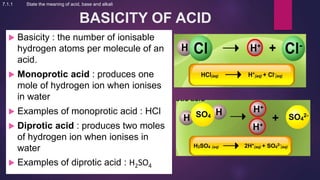
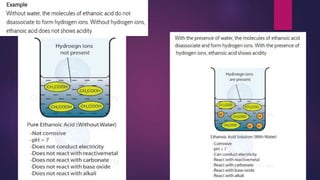
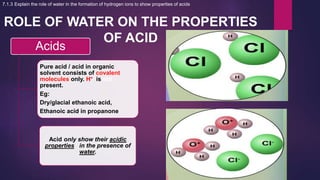
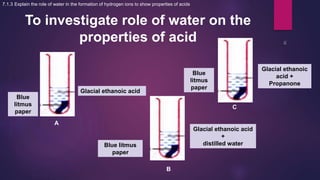
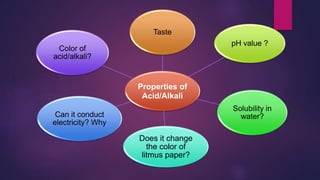
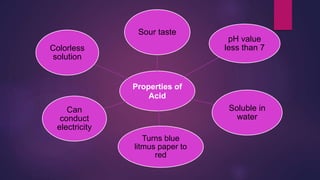
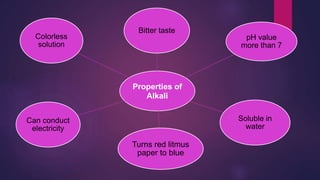
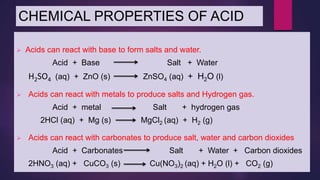
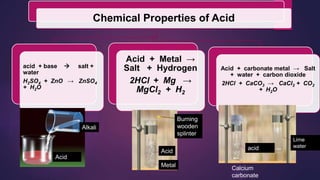
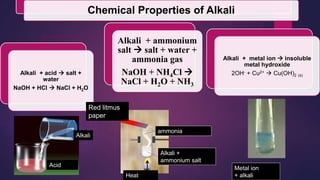
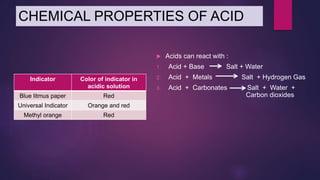
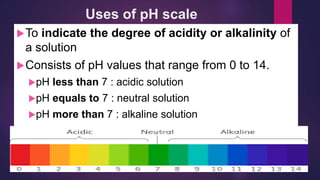
The document discusses acids and bases. It defines acids as compounds that ionize in water to produce hydrogen ions, and bases as compounds that react with acids to produce salts and water. Alkalis are bases that ionize in water to produce hydroxide ions. Water is necessary for acids and alkalis to exhibit their properties, as it allows them to dissociate into ions. The document also outlines the chemical properties of acids and bases, such as their reactions with each other, metals, and carbonates to produce salts, water, hydrogen gas or carbon dioxide. Common uses of acids and bases in daily life are also mentioned.















![leenl@
chemf4
29
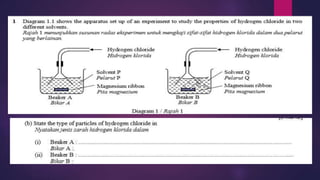
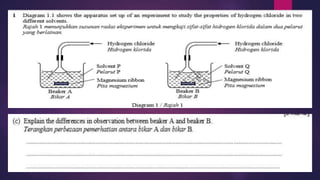
Explain the following:
A solution of hydrogen chloride in toluene does not react
with marble whereas a solution of hydrogen chloride in
water does.
[6 marks]
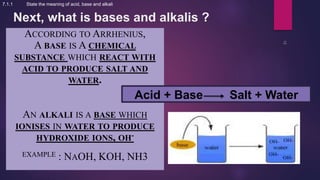
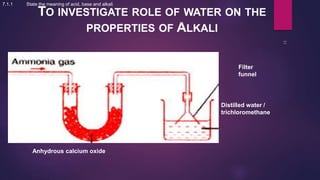
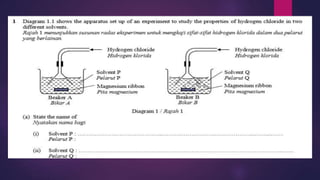
7.1.1 State the meaning of acid, base and alkali](https://image.slidesharecdn.com/c7acidsbases-190825074610/85/FORM-4-CHAPTER-7-ACIDS-AND-BASES-29-320.jpg)

















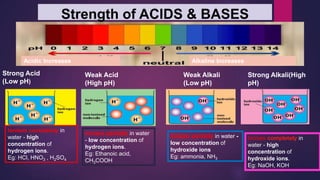


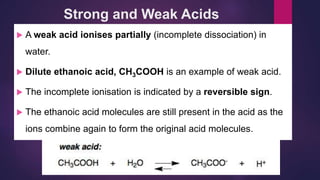
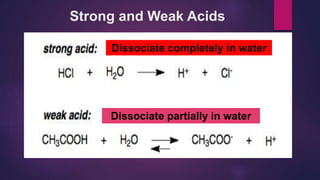



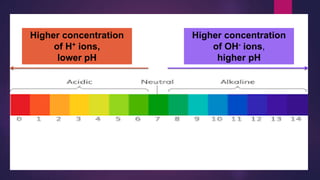












![Try this!
The molarity of a bottle of nitric acid, HNO3 solution
is 2.0 mol dm-3. What is the concentration of the
solution in g dm-3?
[Relative atomic masses : H, 1; N, 14; O, 16]
*Refer to concentration formula.
What information do u have?](https://image.slidesharecdn.com/c7acidsbases-190825074610/85/FORM-4-CHAPTER-7-ACIDS-AND-BASES-82-320.jpg)
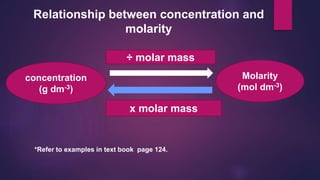
![Concentrations of acids and alkalis
M = Concentration in mol dm-3 [Molarity]
V = Volume in cm3
1000 cm3 = 1 dm3
No. of mol
MV_
1000
84](https://image.slidesharecdn.com/c7acidsbases-190825074610/85/FORM-4-CHAPTER-7-ACIDS-AND-BASES-84-320.jpg)


![leenl@
chemf4
87
1. What is the mass of sodium carbonate required to dissolve
in water to prepare a 200cm3 solution that contains 50
g/dm3?
Volume of solution = 200 cm3 = 0.2 dm3
Concentration (g/dm3)
=[(mass of Na2CO3 dissolved (g)] ÷ [volume of solution (dm3)]
Hence, mass of Na2CO3 required
= concentration (g/dm3) x volume of solution (dm3)
= 50 g/dm3 x 0.2 dm3
= 10 g](https://image.slidesharecdn.com/c7acidsbases-190825074610/85/FORM-4-CHAPTER-7-ACIDS-AND-BASES-87-320.jpg)
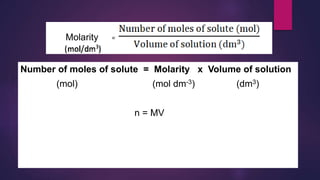
![leenl@
chemf4
88
Calculation involving concentrations of solutions in mol/dm3
Molarity, M (mol/dm3) = No. of moles of solute, n (mol)
Volume of solution, V (dm3)
No. of moles,n (mol) = Molarity (M) x Volume (V) dm3
OR
No. of moles (mol) = Molarity (M) x [Volume (V) / 1000 cm3 ]
= MV/1000](https://image.slidesharecdn.com/c7acidsbases-190825074610/85/FORM-4-CHAPTER-7-ACIDS-AND-BASES-88-320.jpg)








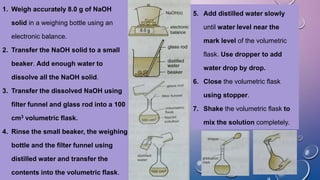
![Preparing 100 cm3 of 1.0 mol/dm3
aqueous sodium hydroxide solution
Mass of NaOH required to prepare 100cm3 of 1.0 mol/dm3
= [(MV)/1000] x RMM of NaOH
= [(10 x 100)/1000] x (23 + 16 + 1)
= 4 g
4.0 g of NaOH is weighed accurately
4.0 g of NaOH is transferred to a small beaker. Distilled water is added to dissolve the
solid NaOH.](https://image.slidesharecdn.com/c7acidsbases-190825074610/85/FORM-4-CHAPTER-7-ACIDS-AND-BASES-98-320.jpg)