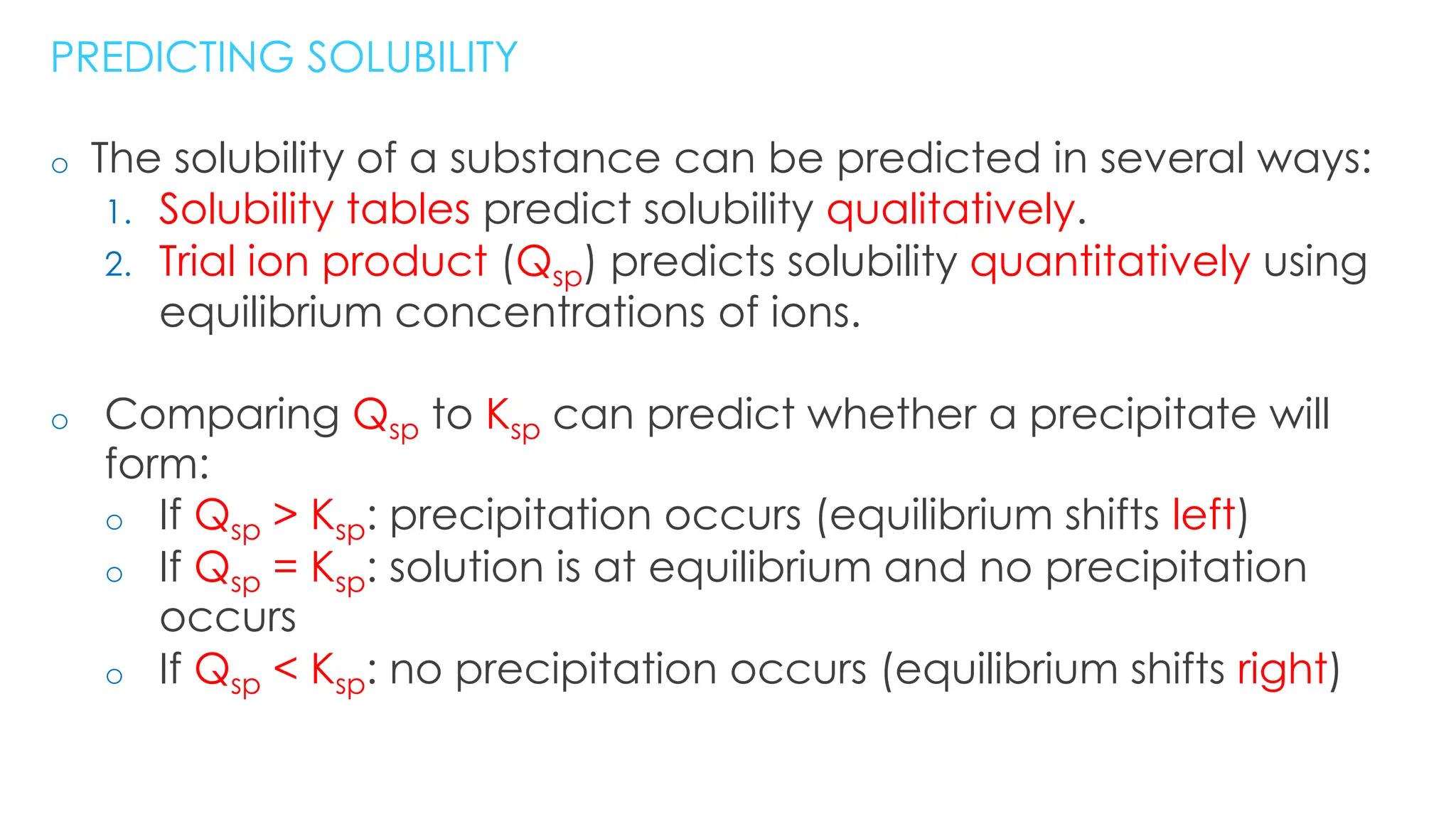
This document discusses solubility, defining key concepts such as molar solubility, saturated solutions, and solubility product constants (ksp). It explains how ionic compounds behave in water, using examples to illustrate concepts of solubility equilibria, the impact of common ions, and factors affecting solubility, including pH. Additionally, it provides calculations for determining ksp and solubilities from various scenarios and emphasizes the importance of these concepts in predicting precipitation reactions.







![Solubility Product
• Equilibrium constant for the dissociation of a solid salt into its aqueous ions:
solubility product, Ksp
• For an ionic solid MnXm, the dissociation reaction is:
MnXm(s) nMm+(aq) + mXn−(aq)
• The solubility product would be
Ksp = [Mm+]n[Xn−]m](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-8-2048.jpg)
![The Solubility Product Constant (Ksp)
Similar to what we have seen for the equilibrium constant, K
e.g. the dissolution of BaSO4
K = [Ba2+(aq)] [SO42-(aq)]
[BaSO4(s)]
Do we include the solid?](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-9-2048.jpg)
![Solubility Product Constant
• Since the [ ] of solid BaSO4 does not change, then it becomes part of
the Ksp.
Therefore, Ksp = [Ba2+
(aq)] [SO4
2-(aq)]
Ksp is calculated using [ ] in mol/L and is unitless
Ksp varies with temperature
Ksp usually reported for slightly soluble compounds](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-10-2048.jpg)


![SOLUBILITY EQUILIBRIA
o Calcium phosphate (Ca3(PO4)2) is one of the salts responsible for kidney
stones. Write the solubility product constant (Ksp) equation for the
dissolution of solid Ca3(PO4)2.
Ca3(PO4)2(s) ⮀ 3 Ca2+
(aq) + 2 PO4
3ˉ
(aq)
Ksp = [Ca2+]3 [PO4
3ˉ]2
o Limestone is composed of calcium carbonate (CaCO3). Acid rain causes the
CaCO3 present in limestone to dissociate into its ions. Write the solubility
product constant (Ksp) equation for this process.
Ca CO3(s) ⮀ Ca2+
(aq) + CO3
2ˉ
(aq)
Ksp = [Ca2+] [CO3
2ˉ]](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-13-2048.jpg)

![CALCULATING Ksp
Example1: USING ION CONCENTRATIONS
A piece of solid zinc hydroxide (Zn(OH)2(s)) is placed in a container of water and sealed. A
chemist determines that the concentrations of aqueous zinc ions and aqueous hydroxide
ions is 2.7 x 10-6 mol/L and 5.4 x 10-6 mol/L, respectively. Calculate the value of Ksp.
❶Write the balanced equation for the dissociation of zinc hydroxide:
Zn(OH)2(s) ⮀ Zn2+
(aq) + 2 OH1–
(aq)
❷Write the Ksp equation and solve for Ksp:
Ksp = [Zn2+][OH1–]2
= (2.7 x 10-6)(5.4 x 10-6)2
= 7.873 x 10-17
= 7.9 x 10-17
❸Write a concluding statement:
The solubility product constant for Zn(OH)2(s) is 7.9 x 10 -17.](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-15-2048.jpg)
![CALCULATING Ksp
Example 2: USING MOLAR SOLUBILITY
It has been determined that the molar solubility of silver chloride
(AgCl(s)) is 1.3 × 10–5 mol/L. What is the value of the solubility-
product constant for this solid?
❶Write the balanced equation for the dissociation of silver chloride:
AgCl (s) ⮀ Ag1+
(aq) + Cl1–
(aq)
❷Use stoichiometry to solve for [Ag1+] and [Cl1–]
Since there is a 1:1:1 in the balanced equation, the ion concentrations are
[AgCl] = [Ag1+] = [Cl1–] = 1.3 x 10-5 mol/L
❸Write a Ksp equation and solve for Ksp:
Ksp = [Ag1+][Cl1–]
= (1.3 x 10-5)(1.3 x 10-5)
= 1.69 x 10-10
= 1.7 x 10-10
❹ Write a concluding statement:
The solubility product
constant for AgCl(s) is
1.7 x 10 -10.](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-16-2048.jpg)
![CALCULATING [EQ]
Example 3: USING Ksp
Calculate the concentration of each ion present and the molar solubility
of solid copper (II) iodate (Cu(IO3)2) if Ksp is 6.9 x 10-8 at 25°C.
❶Write the balanced equation for the dissociation of copper (II) iodate:
Cu(IO3)2(s) ⮀ Cu2+
(aq) + 2 IO3
1–
(aq)
❷Set up an ICE table:
Cu(IO3)2(s) ⮀ Cu2+
(aq) + 2 IO3
1–
(aq)
I -- 0 0
C -- + x + 2x
E -- X 2x](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-17-2048.jpg)
![CALCULATING [EQ]
Example 3 CONT’D: USING Ksp
❹Determine [Cu2+] and [IO3
1–]
and molar solubility of
Cu(IO3)2:
[Cu2+] = x
= 2.6 x 10-3 mol/L
[IO3
1–] = 2x
= 2(2.6 x 10-3)
= 5.12 x 10-3
= 5.1 x 10-3 mol/L
[Cu(IO3)2] = [Cu2+] = x
= 2.6 x 10-3 mol/L](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-18-2048.jpg)






![What is the solubility of silver chloride in g/L ?
AgCl (s) Ag+ (aq) + Cl- (aq)
Ksp = [Ag+][Cl-]
Initial (M)
Change (M)
Equilibrium (M)
0.00
+s
0.00
+s
s s
Ksp = s2
s = Ksp
s = 1.3 x 10-5
[Ag+] = 1.3 x 10-5 M [Cl-] = 1.3 x 10-5 M
Solubility of AgCl = 1.3 x 10-5 mol AgCl
1 L soln
143.35 g AgCl
1 mol AgCl
x = 1.9 x 10-3 g/L
Ksp = 1.6 x 10-10](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-25-2048.jpg)
![If 2.00 mL of 0.200 M NaOH are added to 1.00 L of
0.100 M CaCl2, will a precipitate form?
The ions present in solution are Na+, OH-, Ca2+, Cl-.
Only possible precipitate is Ca(OH)2 (solubility rules).
Is Q > Ksp for Ca(OH)2?
[Ca2+]0 = 0.100 M [OH-]0 = 4.0 x 10-4 M
Ksp = [Ca2+][OH-]2 = 8.0 x 10-6
Q = [Ca2+]0[OH-]0
2= 0.10 x (4.0 x 10-4)2 = 1.6 x 10-8
Q < Ksp No precipitate will form](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-26-2048.jpg)
![What concentration of Ag is required to precipitate
ONLY AgBr in a solution that contains both Br- and Cl- at
a concentration of 0.02 M?
AgCl (s) Ag+ (aq) + Cl- (aq)
Ksp = [Ag+][Cl-]
Ksp = 1.6 x 10-10
AgBr (s) Ag+ (aq) + Br- (aq)Ksp = 7.7 x 10-13
Ksp = [Ag+][Br-]
[Ag+] =
Ksp
[Br-]
7.7 x 10-13
0.020
= = 3.9 x 10-11 M
[Ag+] =
Ksp
[Cl-]
1.6 x 10-10
0.020
= = 8.0 x 10-9 M
3.9 x 10-11 M < [Ag+] < 8.0 x 10-9 M](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-27-2048.jpg)

![Tro, Chemistry: A Molecular Approach 29
Calculating Solubility from Ksp
The Ksp for copper (II) hydroxide is 1.6 x 10-19 at
25˚C. Calculate the solubility, [Cu2+], [OH-].](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-29-2048.jpg)


![The Common Ion Effect and Solubility
The presence of a common ion decreases the
solubility of the salt.
What is the molar solubility of AgBr in (a) pure water
and (b) 0.0010 M NaBr?
AgBr (s) Ag+ (aq) + Br- (aq)
Ksp = 7.7 x 10-13
s2 = Ksp
s = 8.8 x 10-7
NaBr (s) Na+ (aq) + Br- (aq)
[Br-] = 0.0010 M
AgBr (s) Ag+ (aq) + Br- (aq)
[Ag+] = s
[Br-] = 0.0010 + s 0.0010
Ksp = 0.0010 x s
s = 7.7 x 10-10](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-32-2048.jpg)

![The Effect of pH on Solubility
• For insoluble ionic hydroxides, the higher the pH (and
the greater [OH-], the lower the solubility of the ionic
hydroxide
• OH- acts as a common ion,
M(OH)n(s) Mn+(aq) + nOH−(aq)
• For insoluble ionic compounds that contain anions of
weak acids, the lower the pH, the higher the solubility
M2(CO3)n(s) 2 Mn+(aq) + nCO3
2−(aq)
H3O+(aq) + CO3
2− (aq) HCO3
− (aq) + H2O(l)](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-34-2048.jpg)


![PREDICTING SOLUBILITY
Example: Will a precipitate form when 1.0 x 10-3 mol/L of aqueous silver nitrate
(AgNO3) is mixed with a 5.0 x 10-3 mol/L aqueous solution of potassium bromide
(KBr) at 25°C? If so, identify the precipitate. The Ksp for AgBr is 5.4 x 10–13
• Write the balanced equation for the double displacement reaction and
determine any possible precipitates:
AgNO3(aq) + KBr(aq) ⮀ AgBr(s) + KNO3(aq)
• Write a dissociation equation for the precipitate (solid) that may form:
AgBr(s) ⮀ Ag1+
(aq) + Br1–
(aq)
• Write the Qsp expression for the precipitate (AgBr):
Qsp = [Ag1+][Br1–]](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-38-2048.jpg)
![• Find the concentration of each ion ([Ag1+] and [Br1–]
*To do this, write the dissociation equations for each reactant (AgNO3 and KBr) and use
stoichiometry to find each ion’s concentration.
AgNO3(aq) ⮀ Ag1+
(aq) + NO3
1–
(aq) AND KBr(aq) ⮀ K1+
(aq) + Br1–
(aq)
[Ag1+] = [AgNO3] [Br1–] = [KBr]
= 1.0 x 10-3 mol/L = 5.0 x 10-3 mol/L
• Calculate Qsp and compare to Ksp:
*Ksp was given in the question. If it isn’t, look for it in the Solubility Product Constant table.
Qsp = [Ag1+][Br1–]
= (1.0 x 10-3)(5.0 x 10-3)
= 5.0 x 10-6
• Write a concluding statement:
Yes, a precipitate of silver bromide (AgBr(s)) will form.
Qsp > Ksp
(5.0 x 10–6 > 5.4 x 10–13
(precipitation occurs)](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-39-2048.jpg)

![ANSWER
CuNO3(aq) + KI(aq) ⮀ CuI(s) + KNO3(aq)
CuI(s) ⮀ Cu1+
(aq) + I1-
(aq)
Qsp = [Cu1+][I1-]
CuNO3(aq) ⮀ Cu1+
(aq) + NO3
1-
(aq)
KI(aq) ⮀ K1+
(aq) + I1-
(aq)
[Cu1+] = [CuNO3] = 0.015 mol/L
[I1-] = [KI] = 0.075 mol/L](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-41-2048.jpg)
![ANSWER
Qsp = [Cu1+][I1-] *Ksp = 1.3 x 10–12
= (0.015)(0.075)
= 1.125 x 10–3
Qsp > Ksp (1.125 x 10–3 > 1.3 x 10–12), so a precipitate will
form.](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-42-2048.jpg)

![Predicting Precipitation
• Calculation:
• [Pb2+
] = (20.0 mL x 0.025 M)/(50.0 mL) = 0.010 M
• [Cl-
] = (30.0 mL x 0.10 M)/(50.0 mL) = 0.060 M
• Qsp = [Pb2+
][Cl-
]2
= (0.010 M)(0.060 M)2
• = 3.6 x 10-5
• Qsp > Ksp ➔ precipitate of PbCl2 will form.](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-44-2048.jpg)

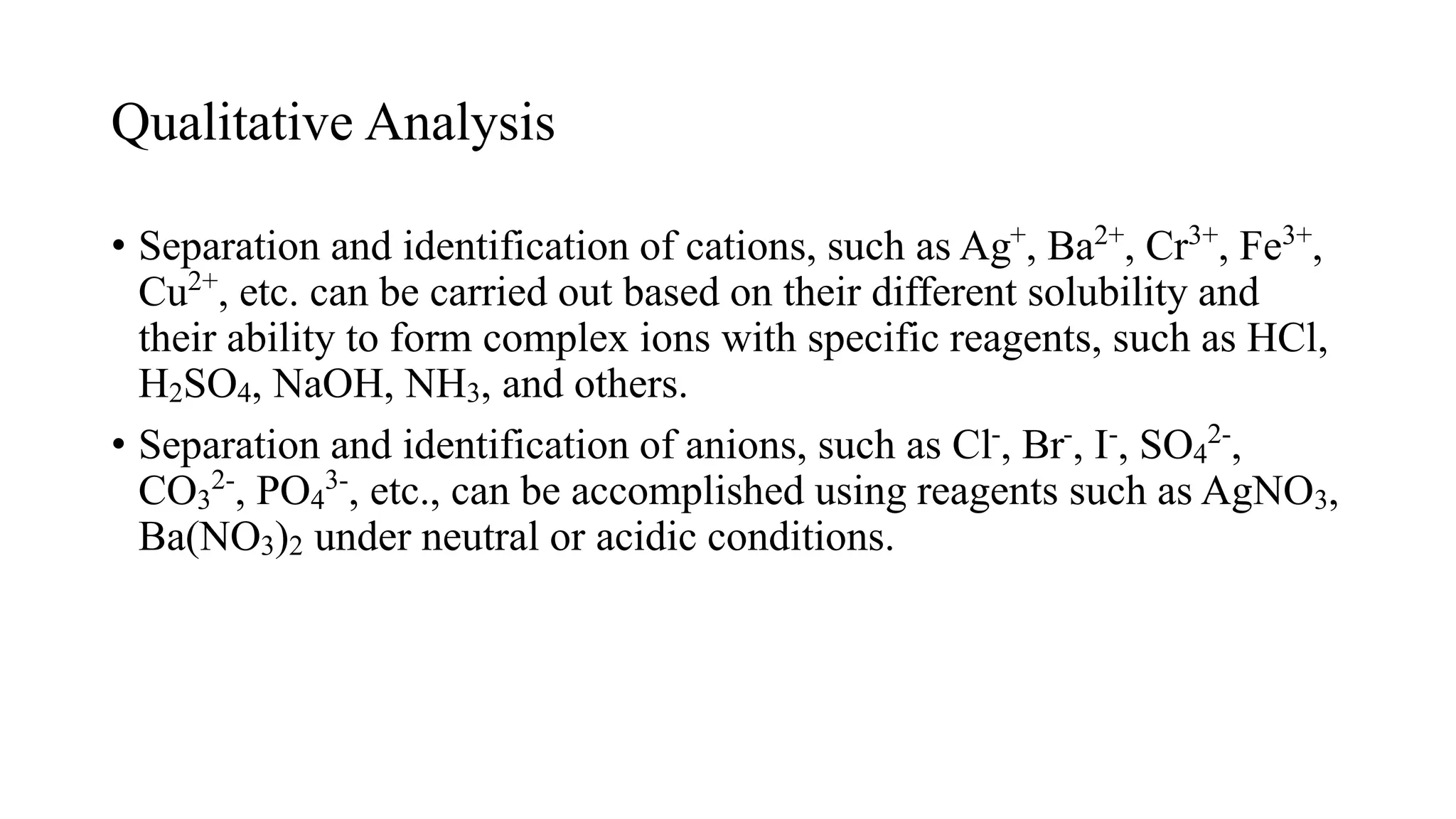

![Separation of Cu2+ and Hg2+ from Ni2+ and
Mn2+ using H2S
• At a low pH, [S2–] is relatively low and only the very
insoluble HgS and CuS precipitate.
• When OH– is added to lower [H+], the value of [S2–]
increases, and MnS and NiS precipitate.](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-49-2048.jpg)




![Complex Ion Equilibria
• Complex ions are ions consisting central metal ions and ligands
covalently bonded to the metal ions;
• Ligands can be neutral molecules such as H2O, CO, and NH3, or
anions such as Cl-
, F-
, OH-
, and CN-
;
• For example, in the complex ion [Cu(NH3)4]2+
, four NH3 molecules
are covalently bonded to Cu2+
.](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-54-2048.jpg)
![Formation of Complex Ions
• In aqueous solutions, metal ions form complex ions
with water molecules as ligands.
• If stronger ligands are present, ligand exchanges occur
and equilibrium is established.
• For example:
Cu2+
(aq) + 4NH3(aq) ⇌ [Cu(NH3)4]2+
(aq)
13
4
3
2
2
4
3
f 10
x
1.1
]
NH
][
Cu
[
]
)
[Cu(NH
=
= +
+
K](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-55-2048.jpg)
![Stepwise Formation of Complex Ion
• At molecular level, ligand molecules or ions combine with
metal ions in stepwise manner;
• Each step has its equilibrium and equilibrium constant;
• For example:
(1) Ag+
(aq) + NH3(aq) ⇌ Ag(NH3)+
(aq)
(2) Ag(NH3)+
(aq) + NH3(aq) ⇌ Ag(NH3)2
+
(aq);
3
3
3
f1 10
x
2.1
]
][NH
[Ag
]
)
[Ag(NH
=
= +
+
K
3
3
3
2
3
2
f 10
x
8.2
]
][NH
)
[Ag(NH
]
)
[Ag(NH
=
= +
+
K](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-56-2048.jpg)
![Stepwise Formation of Complex Ion
Individual equilibrium steps:
(1) Ag+
(aq) + NH3(aq) ⇌ Ag(NH3)+
(aq); Kf1 = 2.1 x 103
(2) Ag(NH3)+
(aq) + NH3(aq) ⇌ Ag(NH3)2
+
(aq); Kf2 = 8.2 x 103
Combining (1) and (2) yields:
• Ag+
(aq) + 2NH3(aq) ⇌ Ag(NH3)2
+
(aq);
7
f2
f1
2
3
2
3
f 10
x
1.7
x
]
][NH
[Ag
]
)
[Ag(NH
=
=
= +
+
K
K
K](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-57-2048.jpg)
![Stepwise complex ion formation for Cu(NH3)4
2+
Individual equilibrium steps:
1. Cu2+(aq) + NH3(aq) ⇌ Cu(NH3)2+(aq); K1 = 1.9 x 104
2. Cu(NH3)2+(aq) + NH3(aq) ⇌ Cu(NH3)2
2+(aq); K2 = 3.9 x 103
3. Cu(NH3)2
2+(aq) + NH3(aq) ⇌ Cu(NH3)3
2+(aq); K3 = 1.0 x 103
4. Cu(NH3)3
2+(aq) + NH3(aq) ⇌ Cu(NH3)4
2+(aq); K4 = 1.5 x 102
Combining equilibrium:
• Cu2+(aq) + 4NH3(aq) ⇌ Cu(NH3)4
2+(aq);
• Kf = K1 x K2 x K3 x K4 = 1.1 x 1013
]
][NH
[Cu
]
)
[Cu(NH
4
3
2
2
4
3
f +
+
=
K](https://image.slidesharecdn.com/solubilityequilibria-240313054941-b63e211d/75/Solubility-and-Solubility-Product-Constant-58-2048.jpg)


