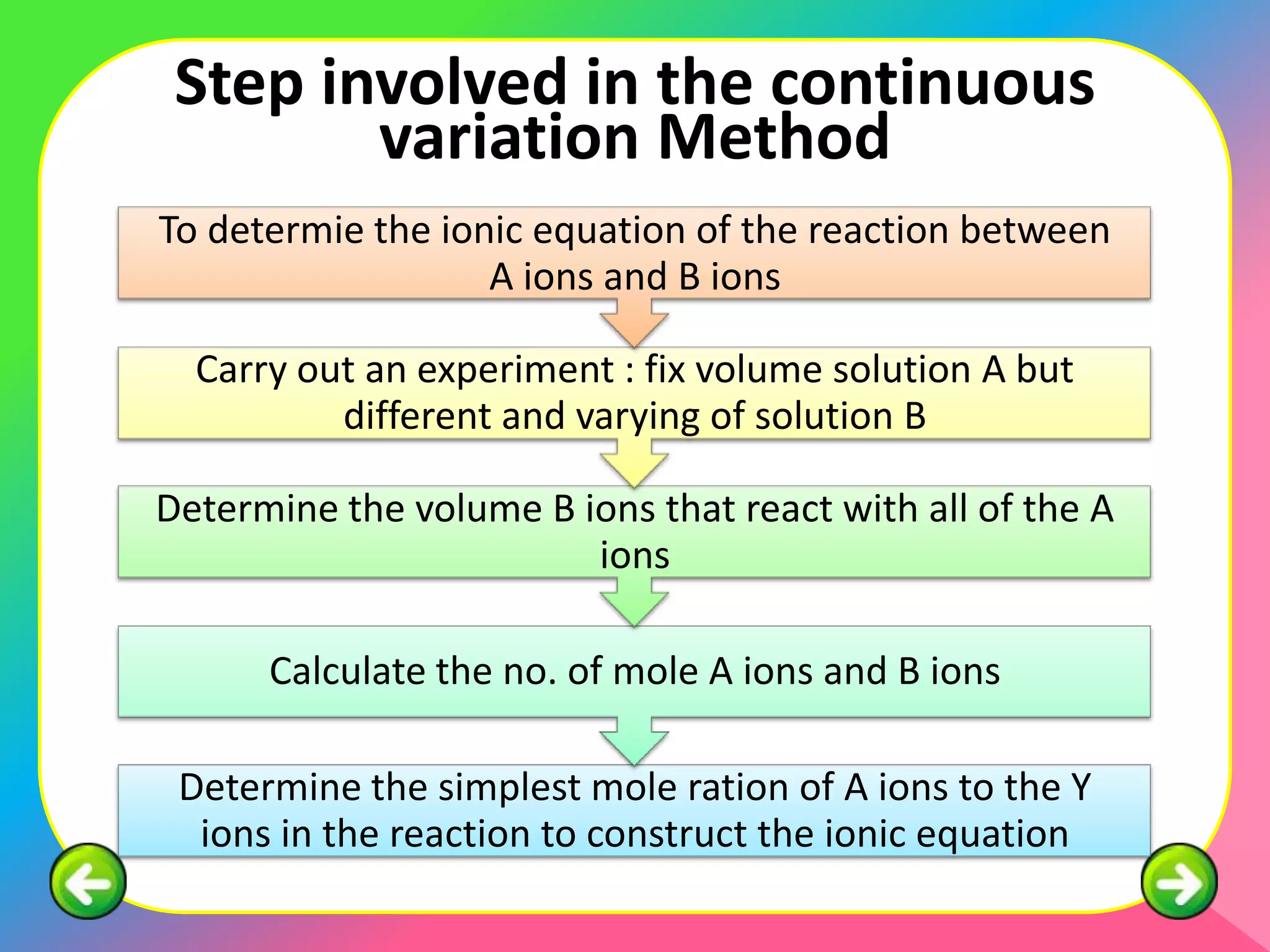
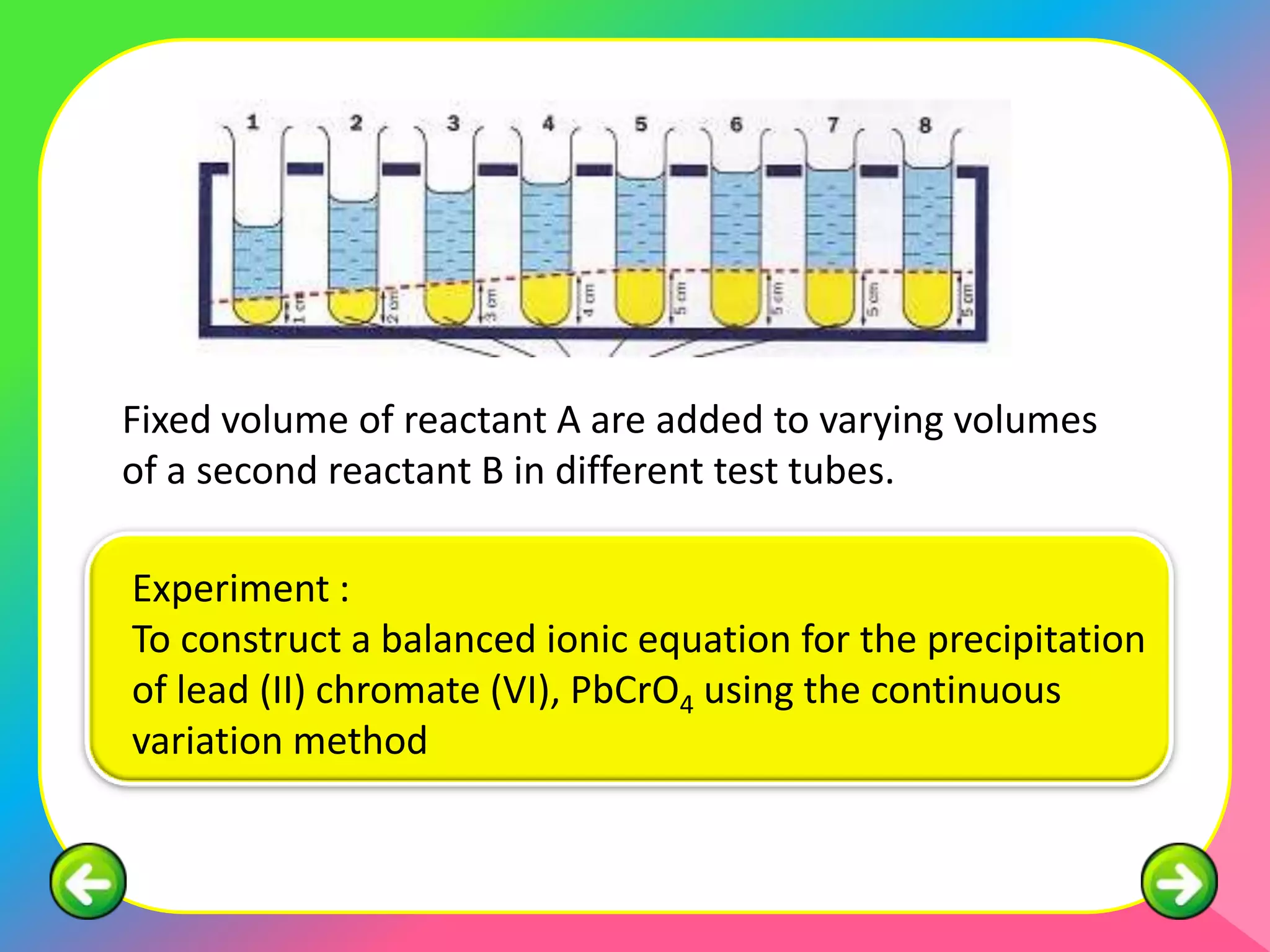
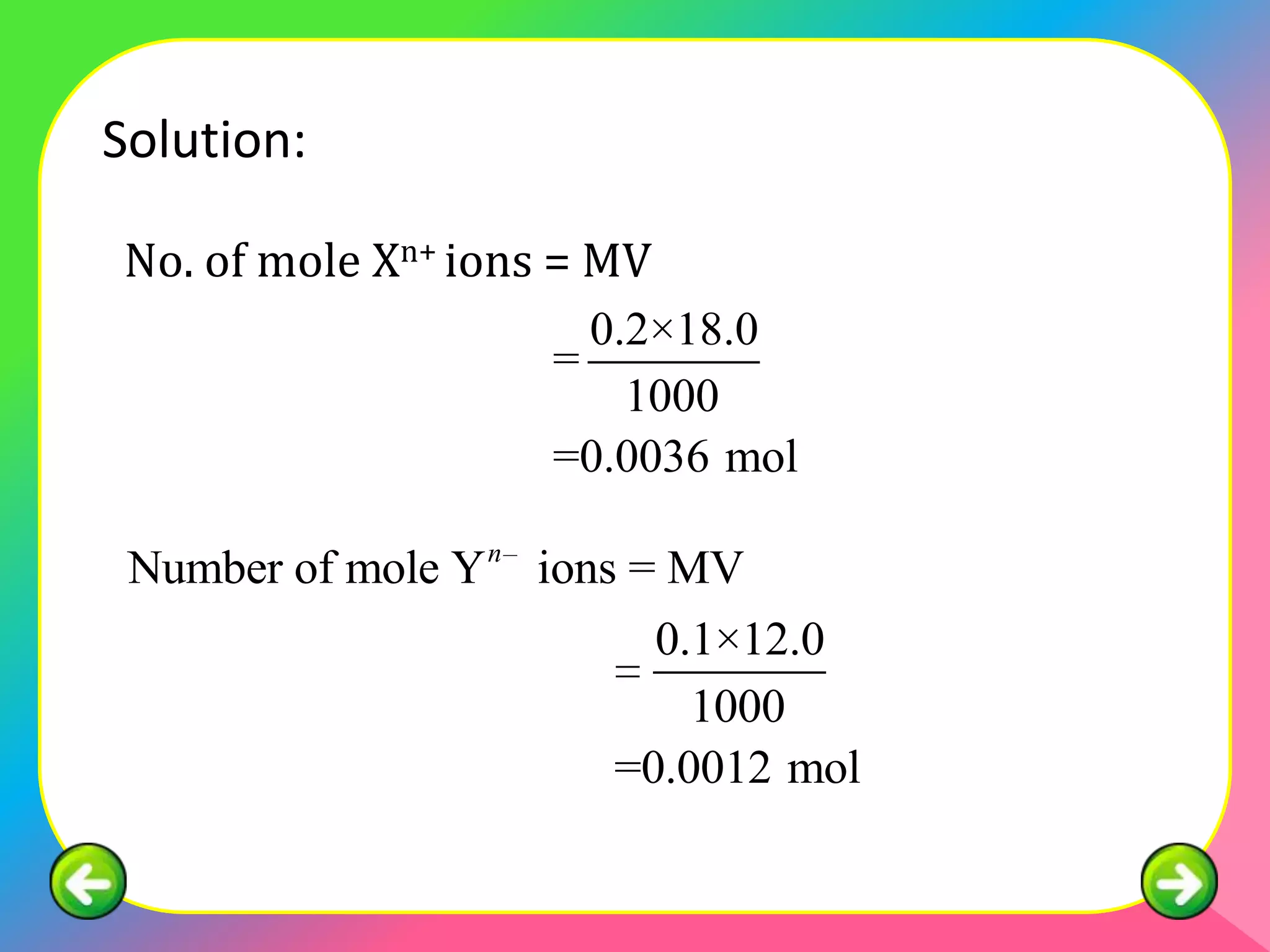
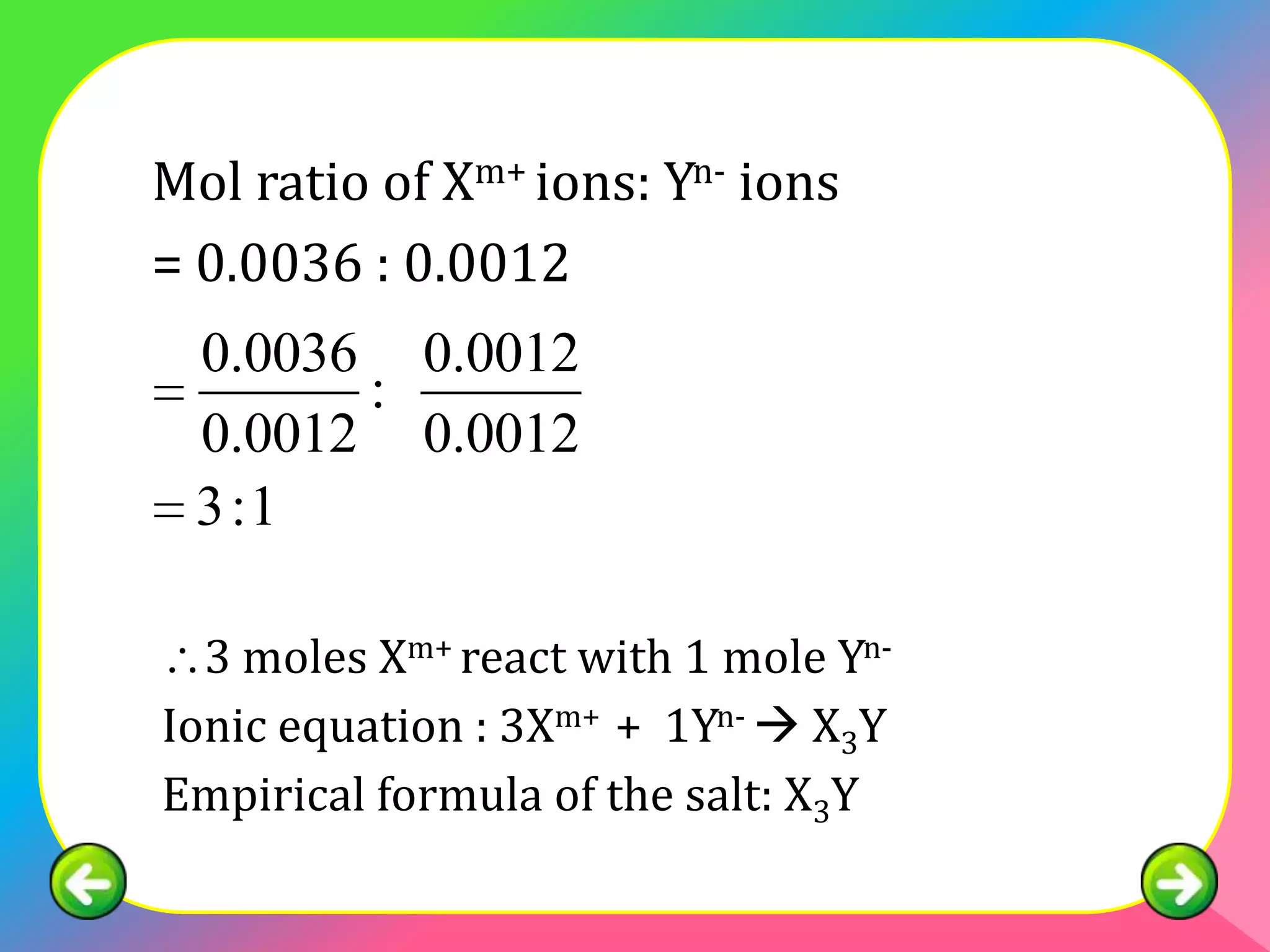
The document describes the continuous variation method used to construct ionic equations for insoluble salts. It involves carrying out experiments where the volume of one reactant is kept fixed while the volume of the other reactant is varied. The mole ratio of the reactants that results in all of the first reactant reacting indicates the ratio in the ionic equation. An example using lead (II) chromate (PbCrO4) precipitation is provided to illustrate the method.