This document outlines the key objectives and concepts around acid-base equilibria, including:
- Defining strong and weak acids/bases using Bronsted-Lowry theory and discussing conjugate acid-base pairs
- Explaining the pH scale and relating pH, pOH, Ka, and pKa values
- Describing how to calculate the pH of strong acids/bases from their concentrations and vice versa
- Discussing how weak acids only partially dissociate in solution according to their acid dissociation constant (Ka)
- Demonstrating calculations for finding the pH of a solution of a weak acid using its Ka value








![The pH scale
The relative strengths of solutions, such as acids,
can be compared by measuring the
concentration of hydronium (or hydrogen) ions
The pH of a solution is the negative logarithm to
base 10 of the concentration of the H3O+ (or H +)
ions in the solution.
pH = −log10 [H3O+], when [H3O+] is given in mol
dm−3
And
[H3O+] = 10-pH](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-12-320.jpg)
![pH and pOH
The concentration of the hydroxide ion can be
measured similarly using a pOH scale:
pOH = −log10 [OH-], when [OH-] is given in mol
dm−3
And,
[OH-] = 10-pOH](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-13-320.jpg)
![pH and pOH
Water itself is slightly ionized giving a small amount of
hydronium and hydroxide ions:
2H2O(l) ↔ H3O+(aq) + OH– (aq)
The [H3O+] in water at 25oC is 10-7 moldm−3
Therefore, pH = −log10 [H3O+]
= −log10 [10-7]
= 7
The [OH-] in water at 25oC is 10-7 moldm−3
Therefore, pOH = −log10 [OH-]
= −log10 [10-7]
= 7
Thus pH + pOH = 14](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-14-320.jpg)
![Calculating the pH of a strong acid
� Since strong acids are fully
ionized in aqueous solution, the
[H3O+] can be found using
stoichiometry
� Example 1
� What is the pH of 0.1 mol dm-3
hydrochloric acid (HCl)?
� Solution
� Since hydrochloric acid is a strong acid, it ionizes
fully in solution:
� HCl(aq) → H+(aq) + Cl-(aq)
� HCl is a monoprotic acid. 1 mol HCl splits up into 1
mol of H+ and 1 mole of Cl-. The concentration of
hydrogen ions is therefore exactly the same as the
concentration of the acid.
� [H+] = 0.1
� pH = -log [H+]
= -log [0.1]
= 1](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-15-320.jpg)
![Calculating the pH of strong acids
� Example 2
� What is the pH of 0.01mol dm-3 sulphuric acid (H2SO4)?
� Solution
� H2SO4(aq) → 2H+
(aq) + SO4
2-
(aq)
� Sulphuric acid is a diprotic acid so, 1 mole of acid produces 2 moles of H+
� [H+] = 2*0.01 = 0.02M
� pH = -log [H+]
= -log [0.02]
= 1.7](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-16-320.jpg)
![Finding The Concentration Of A Strong
Acid From Its pH
� Problem
� What is the concentration of some hydrochloric acid whose pH is 1.60?
� Solution
� HCl(aq) → H+(aq) + Cl-(aq)
� [H+] = 10-pH
= 10-1.60
= 0.025 M (mol dm-3)
HCl is strongly monoprotic so 1 mol of acid ionizes to give 1 mol of hydrogen ion (H+)
� Therefore [HCl] = 0.025 M](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-17-320.jpg)
![Finding The Concentration Of A Strong
Acid From Its pH
� Problem
� What is the concentration of sulphuric acid (H2SO4) if its pH is 1?
� Solution
� H2SO4(aq) → 2H+(aq) + SO4
2-(aq)
� [H+] = 10-pH
= 10-1
= 0.1 M
� H2SO4 is strongly diprotic, 1 mol of acid ionizes to give 2 moles of H+
� [H2SO4] = 0.1/2 = 0.05M](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-18-320.jpg)

![Calculating pH of strong bases
� A base in this context is something which combines with hydrogen ions and a
strong base is one which is fully ionized in solution.
� To calculate the pH of a strong base it is necessary to determine the [OH-] in
moldm-3 and then determine the pOH.
� From here the pH can then be determined using pH = 14 - pOH](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-20-320.jpg)
![Calculating the pH of strong bases
� Problem
� What is the pH of 0.1 M sodium hydroxide solution (NaOH)?
� Solution
� NaOH (s) → Na+(aq) + OH-(aq)
� 1 mole of NaOH gives 1 mole of OH- in solution, so the [OH-] is also 0.1 M
� pOH = −log10 [OH-]
= −log10 [0.1]
= 1
pH = 14 – pOH
= 14 – 1
= 13](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-21-320.jpg)
![Calculating pH of strong bases
� Problem
� What is the pH of 0.015M calcium hydroxide, Ca(OH)2 solution?
� Solution
� Ca(OH)2 (s) → Ca2+(aq) + 2OH-(aq)
� 1 mole Ca(OH)2 gives 2 moles of OH-, so the [OH-] is 2 * 0.015 = 0.03M
� pOH = −log10 [OH-]
= −log10 [0.03]
= 1.5
pH = 14 – pOH
= 14 – 1.5
= 12.5](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-22-320.jpg)
![Finding The Concentration Of A Strong
Base From Its pH
� Problem
� What is the concentration of potassium hydroxide solution, KOH, if its pH is 12.8?
� Solution
� KOH (s) → K+(aq) + OH-(aq)
� pOH = 14 – pH
= 14 – 12.8
= 1.2
� [OH-] = 10-pOH
= 10-1.2
= 0.06M
Since 1 mol KOH produces 1 mol OH-,
[KOH] = 0.06M](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-23-320.jpg)
![Finding The Concentration Of A Strong
Base From Its pH
� Problem
� What is the concentration of barium hydroxide solution, Ba(OH)2, if its pH is 12?
� Solution
� Ba(OH)2 (s) → Ba2+(aq) + 2OH-(aq)
� pOH = 14 – pH
= 14 – 12
= 2
� [OH-] = 10-pOH
= 10-2
= 0.01M
Since 1 mol Ba(OH)2 produces 2 mol OH-,
[Ba(OH)2] = 0.01M/2
=0.005M](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-24-320.jpg)

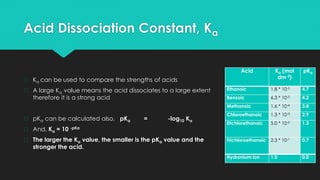
![Acid Dissociation Constant, Ka
� Weak acids dissociate only to a small extent in aqueous sol’n until equilibrium is achieved
� We can write an equilibrium expression for this reaction, called the acid dissociation
constant (Ka)
� Example: ethanoic acid dissolves in water giving,
� CH3COOH (aq) ↔ CH3COO- (aq) + H+(aq)
� Ka = [CH3COO- (aq)] [H+(aq)]
[CH3COOH (aq)]
NB. Since water is the solvent, it is in large excess and is excluded from the equation](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-26-320.jpg)

![Calculating the pH of a weak acid
� Therefore,
� Ka = [C2H5COO-][H+] = (x)(x)
[C2H5COOH]
0.2 - x
� For a weak acid, we assume that since
the dissociation is so little, 0.2-x is
approx. equal to 0.2. So,
� Ka = (x)2
0.2
� Ka = 10 –pKa = 10 -4.82
= 1.513 x 10 -5
� Therefore,
� 1.513 x 10 -5 = x2/0.2
� x = √(1.513 x 10 -5 * 0.2)
� Thus,
� [H+], x = 1.740 x 10 -3
� Hence,
� pH = - log[H+]
= - log (1.740 x 10 -
3)
= 2.76](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-29-320.jpg)
![Calculating Ka for a weak acid
� Problem
� A solution of ethanoic acid of concentration
0.100 mol dm-3 has a pH of 2.88. Calculate
the value of pKa
� Solution
� CH3COOH (aq) ↔ CH3COO- (aq) + H+(aq)
� Ka = [CH3COO-][H+]
[CH3COOH]
� Since ethanoic acid is a weak acid, then
[CH3COOH]initial = [CH3COOH]eq = 0.100 mol
dm-3
� Since pH = 2.88, then
� [H3O+] = 10-pH
= 10 -2.88
= 1.32 x 10-3moldm-3
� Ka = [CH3COO-][H+]
[CH3COOH]
= (1.32 x 10-3moldm-3)(1.32 x 10-3moldm-
3)
0.100 mol dm-3
= 1.74 x 10-3 moldm-3
� pKa = -log Ka
= 4.76](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-30-320.jpg)
![Base Dissociation Constant, Kb
� Kb can be used to measure the strengths of bases
� We can write an equation for the dissociation of a
base and deduce the equilibrium expression
� B (aq) + H2O (l) ↔ BH+
(aq) + OH-
(aq)
� Kb = [BH+
(aq)][OH-
(aq)]
[B (aq)]
� pKb = -log Kb
� And, Kb = 10 –pKb
� The stronger the base, the larger the Kb and hence
the smaller the pKb value.
Example: Ammonia reacts with water
accepting a proton:
NH3(aq) + H2O (l) ↔ NH4
+
(aq) + OH–
(aq)
Kb = [NH4
+
(aq)] [OH–
(aq)]
[NH3(aq)]](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-31-320.jpg)
![Ionic Product of Water, Kw
� Liquid water dissociates to a very small amount extent. As this equilibrium is established,
we can write an equilibrium expression:
� H2O (l) ↔ H+(aq) + OH-(aq)
� Kc =[H+][OH-]/[H2O]
� Since the amount of water that dissociates is so small, the change is considered negligible
and the [H2O] is considered to be a constant. So a new equilibrium constant (Kw) is set up
because [H2O] is constant!
� Kc [H2O]= [H+ (aq)][OH- (aq)]
� Kw = Kc [H2O]
� Kw=[H+][OH-]
At 298 K (25oC), it has been found that
[H+] = [OH-] = 10-7moldm-3
Kw= (10-7moldm-3)2
Kw has a value of 10-14mol2dm-6
pKw = -logKw
pKw = 14](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-32-320.jpg)
![The relationship between Ka and Kb
� When a monoprotic weak acid,
HA, dissociates, the products of
the reversible reaction are A-, the
conjugate base of HA, and H3O+:
� HA (aq) + H2O (l) ↔ H3O+
(aq) + A-
(aq)
� The expression for the equilibrium
constant, Ka, is
� Ka = [H3O+] [A-]
[HA]
� Since A- is a base, we can also
write the reversible rxn for A- acting
as a base by accepting a proton
from water:
� A-
(aq) + H2O (l) ↔ HA (aq) + OH-
(aq)
� The expression for the equilibrium
constant, Kb, is
� Kb = [HA] [OH-]
[A-]](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-33-320.jpg)
![The relationship between Ka, Kb and Kw
� For the conjugate acid-base pair, if we
multiply Ka for HA with Kb for its
conjugate base, A-, we get:
� Ka . Kb = [H3O+] [A-] * [HA] [OH-]
[HA]
[A-]
= [H3O+][OH-]
= Kw
This relationship is very useful for relating Ka
and Kb for a conjugate acid base pair
� Ka Kb = Kw
= 1 x 10-14 mol2dm-6 at 25oC
� Taking the negative log10
� pKa + pKb = pKw
� pKa + pKb = 14 at 25oC
We can use these formulae to determine Kb (or
pKb) of a weak base given the Ka of the
conjugate acid OR calculate the Ka (or pKa) of
a weak acid given Kb of the conjugate base
https://www.khanacademy.org/science/ap-chemistry/acids-and-bases-ap/acid-base-
equilibria-tutorial-ap/a/relationship-between-ka-and-kb](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-34-320.jpg)

![Finding Kb of a weak base
� Problem
� If the pH of a 0.100 moldm-3 solution
ethylamine is 11.85, what is its basic
dissociation constant, pKb?
� Solution
� C2H5NH2 + H2O ↔ C2H5NH3
+ + OH–
� Kb = [C2H5NH3
+] [OH-]
[C2H5NH2]
� Since pH = 11.85
� pOH = 14.0 – 11.85
= 2.15
� [OH-] = 10-pOH
= 10-2.15
= 7.08 x 10-3mol dm-3
We know that [C2H5NH3+] = [OH-],
and [C2H5NH2] = 0.100 moldm-3
Kb = (7.08 x 10-3 mol dm-3)2
0.100 mol dm-3
= 5.01 x 10-4 mol dm-3
pKb = -log [5.01 x 10-4]
= 3.30](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-36-320.jpg)
![Calculating pH of a weak base
� Problem
� What is the pH of methylamine of
concentration 0.45 moldm-3 given
the pKa of CH3NH3
+ to be 9.25?
� Solution
� pKa + pKb = 14
� pKb = 14 – pKa
= 14 – 9.25
= 4.75
� Kb = 10-pKb
= 10-4.75
= 1.775 x 10-5 moldm-3
� CH3NH2 (aq) + H2O (l) ↔ CH3NH3
+
(aq) + OH-
(aq)
I: 0.45
0 0
E: 0.45-x
x x
� Kb = [CH3NH3
+][OH-] = x2
[CH3NH2]
0.45-x
Since methylamine is a weak base,
Kb = x2/0.45
� 1.775 x 10-5 = x2/0.45
x = √ (1.775 x 10-5 x 0.45)
[OH-] = 2.829 x 10-3 moldm-3
� pOH = −log10 [OH-]
=
−log10 [2.829 x 10-3 ]
= 2.55
� pH = 14 – pOH
= 14 – 2.55
= 11.45](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-37-320.jpg)

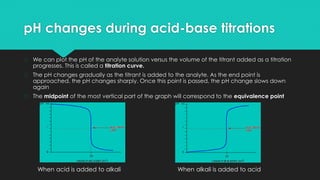
![Acid-Base Indicators
Indicators do not change colour at a
specific [H+] but rather over a narrow
range. This is called the pH range of
the indicator](https://image.slidesharecdn.com/mod23-230404013931-50b3a0ae/85/Mod2-3-Acid-Base-Equilibria-pptx-39-320.jpg)