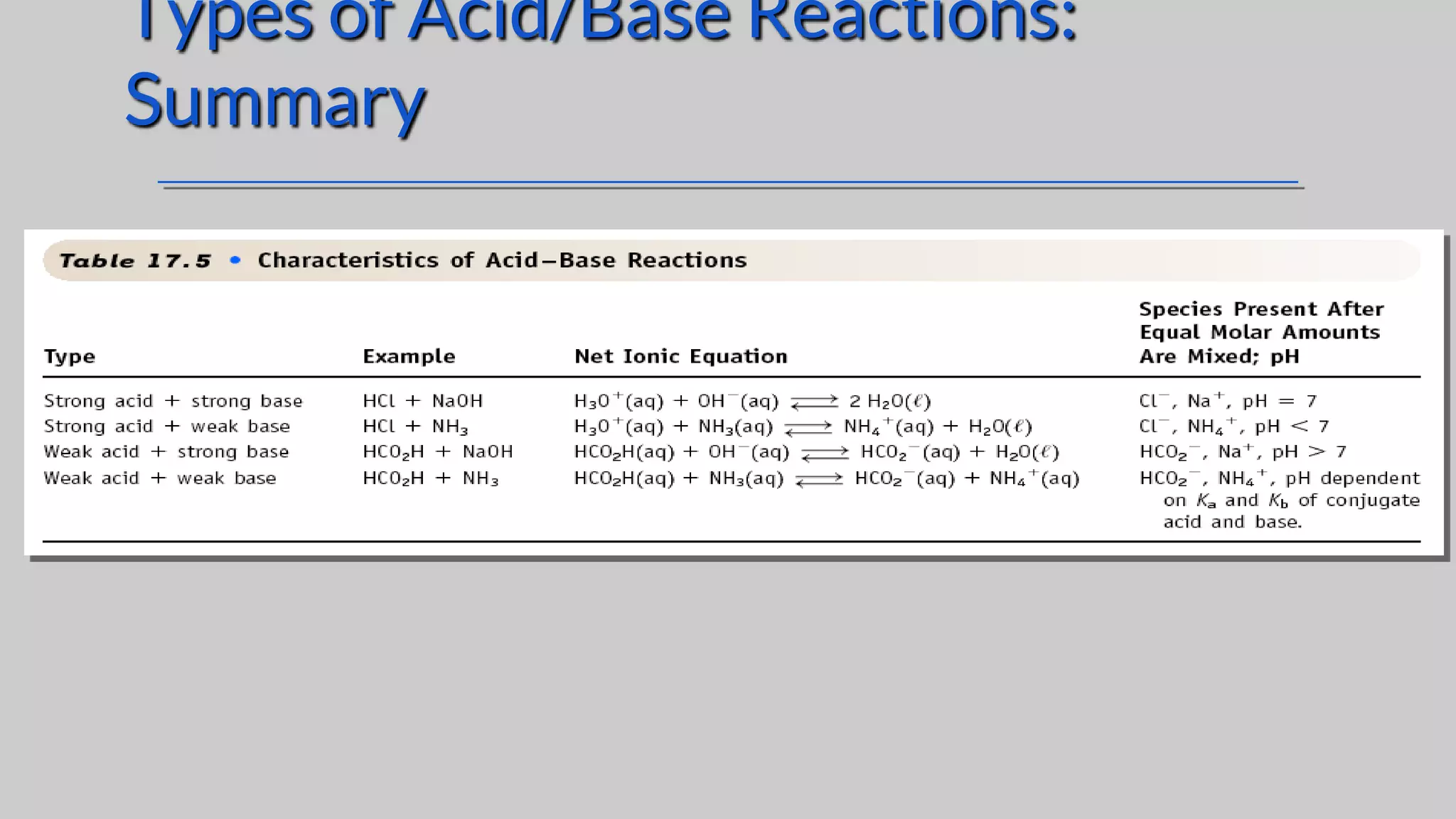
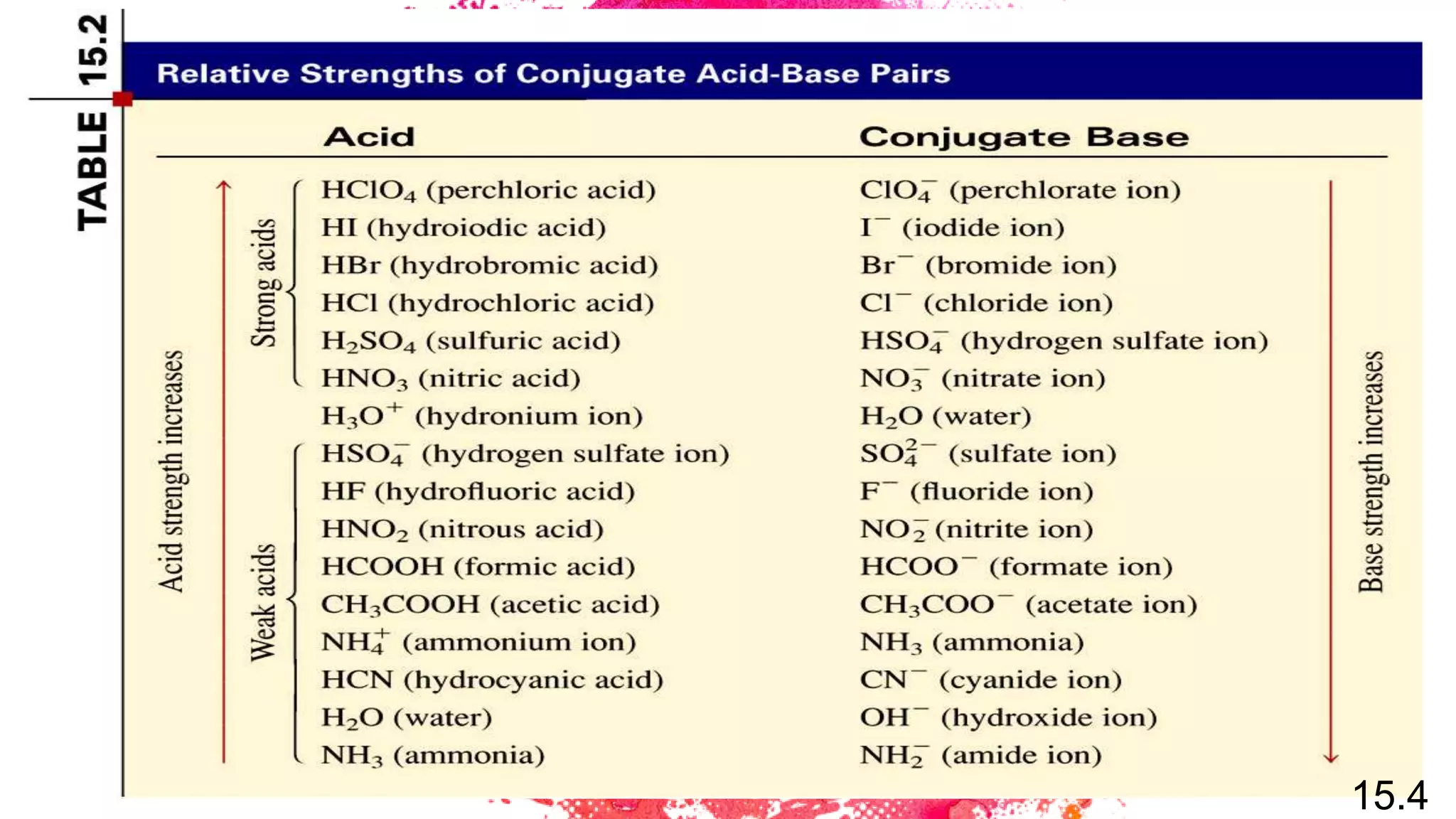
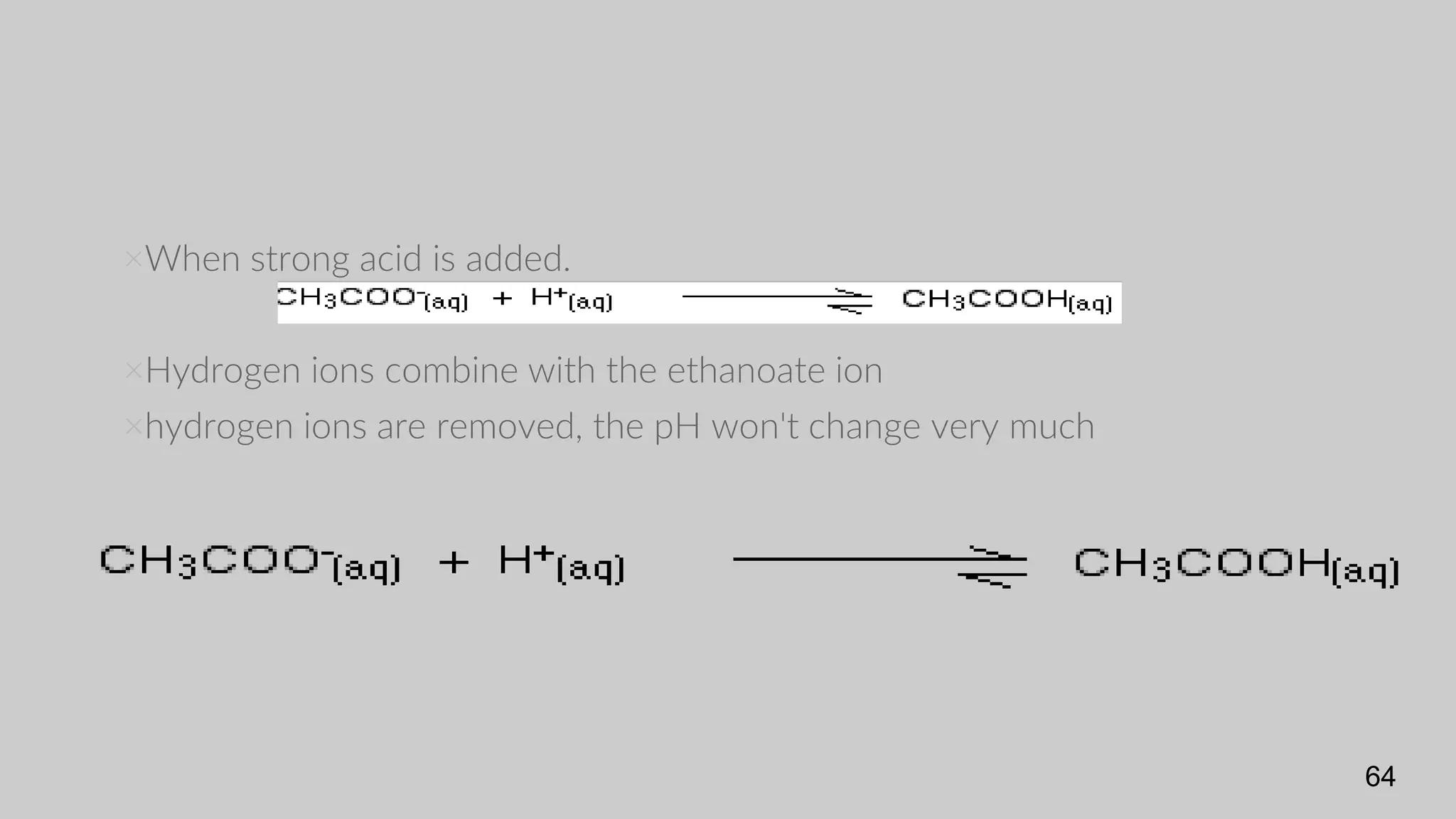
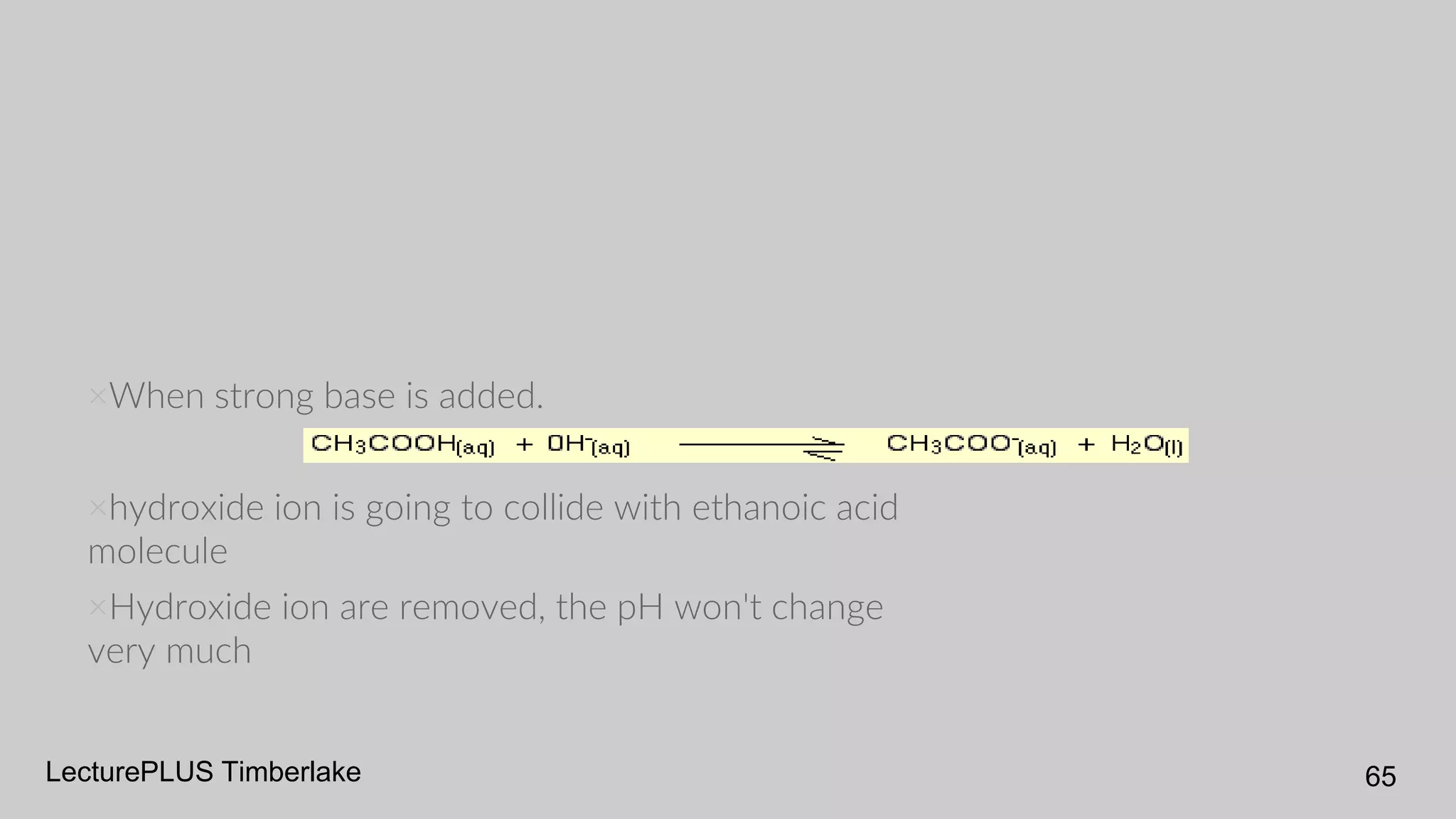
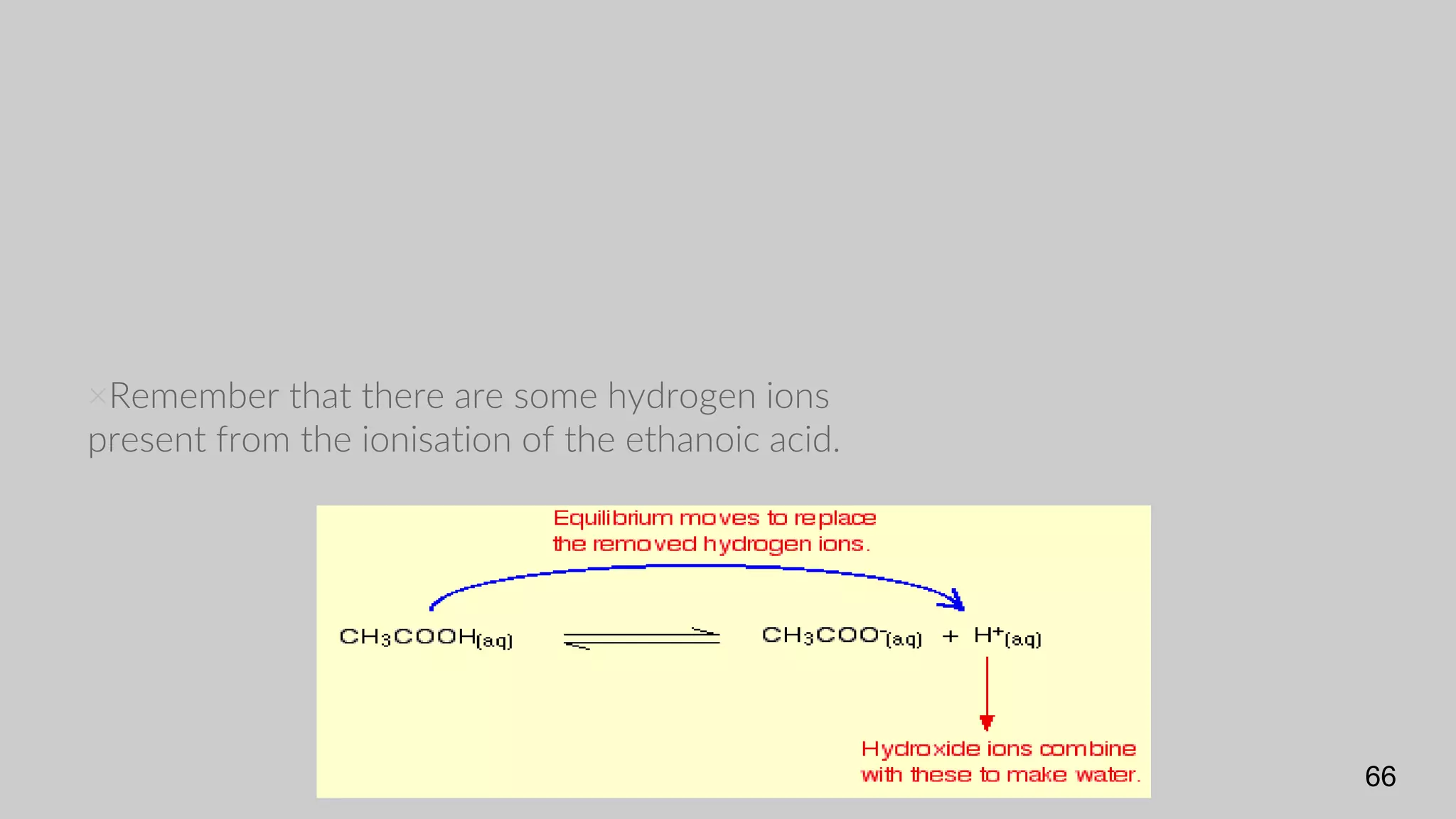
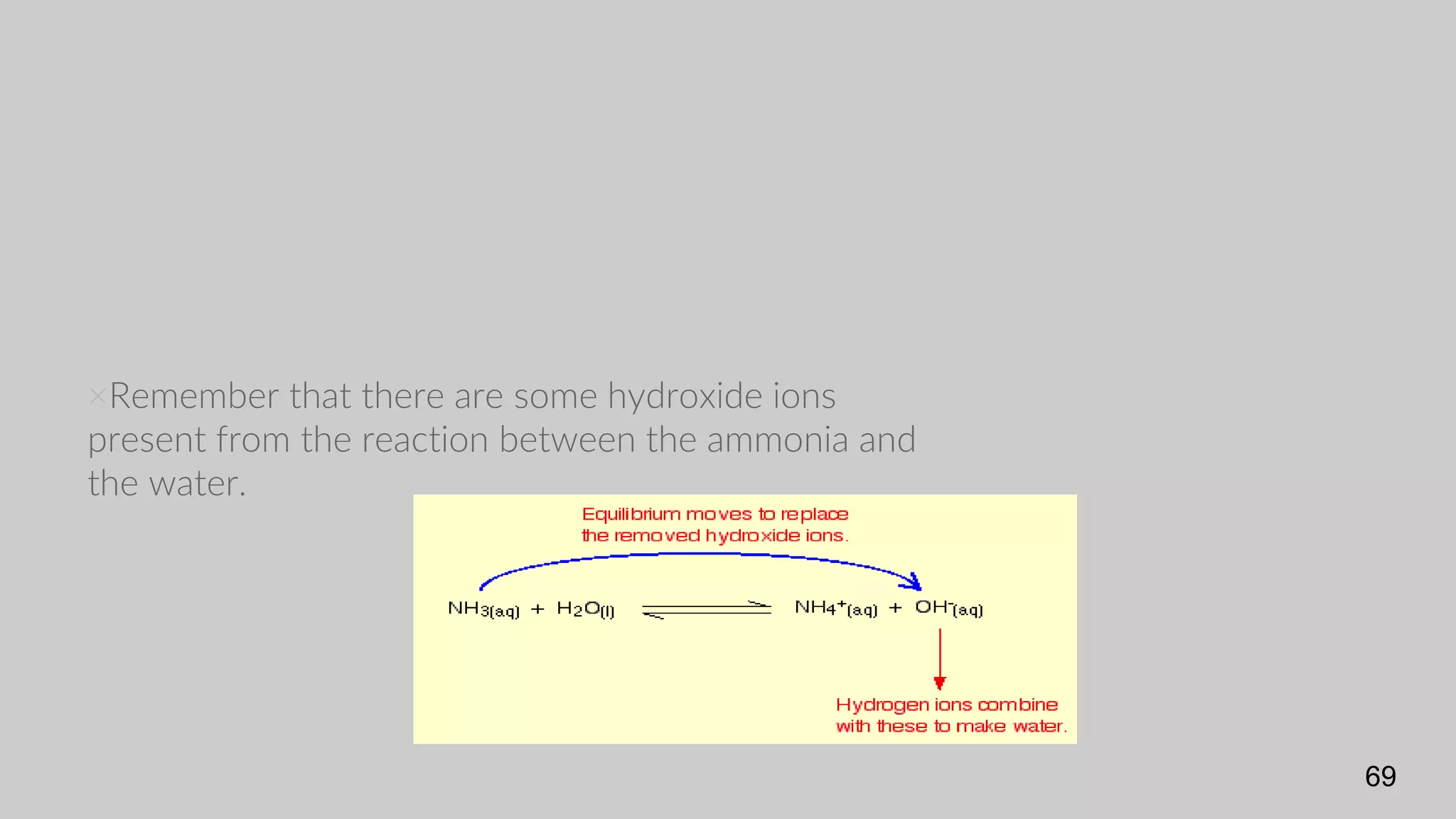
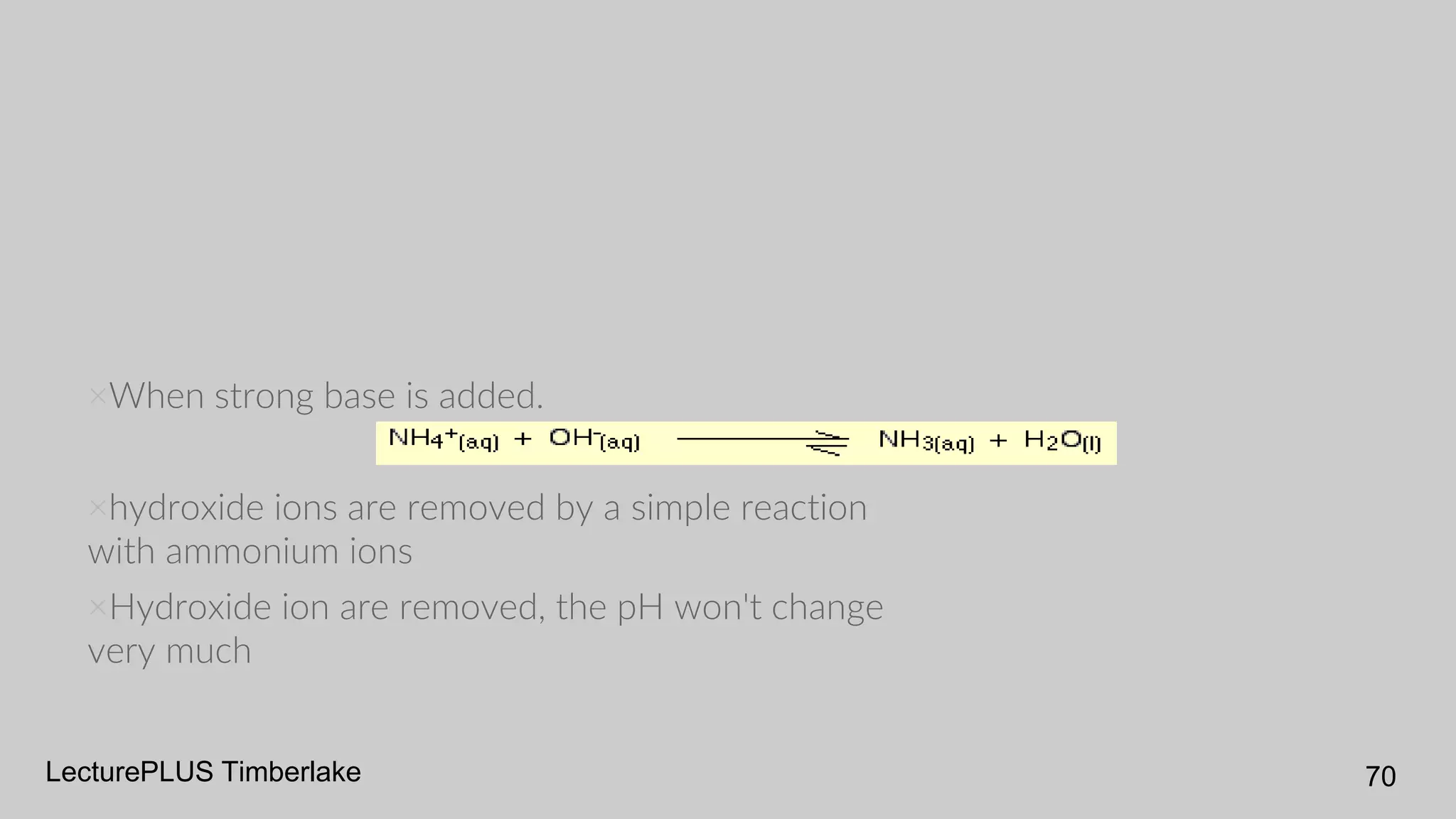
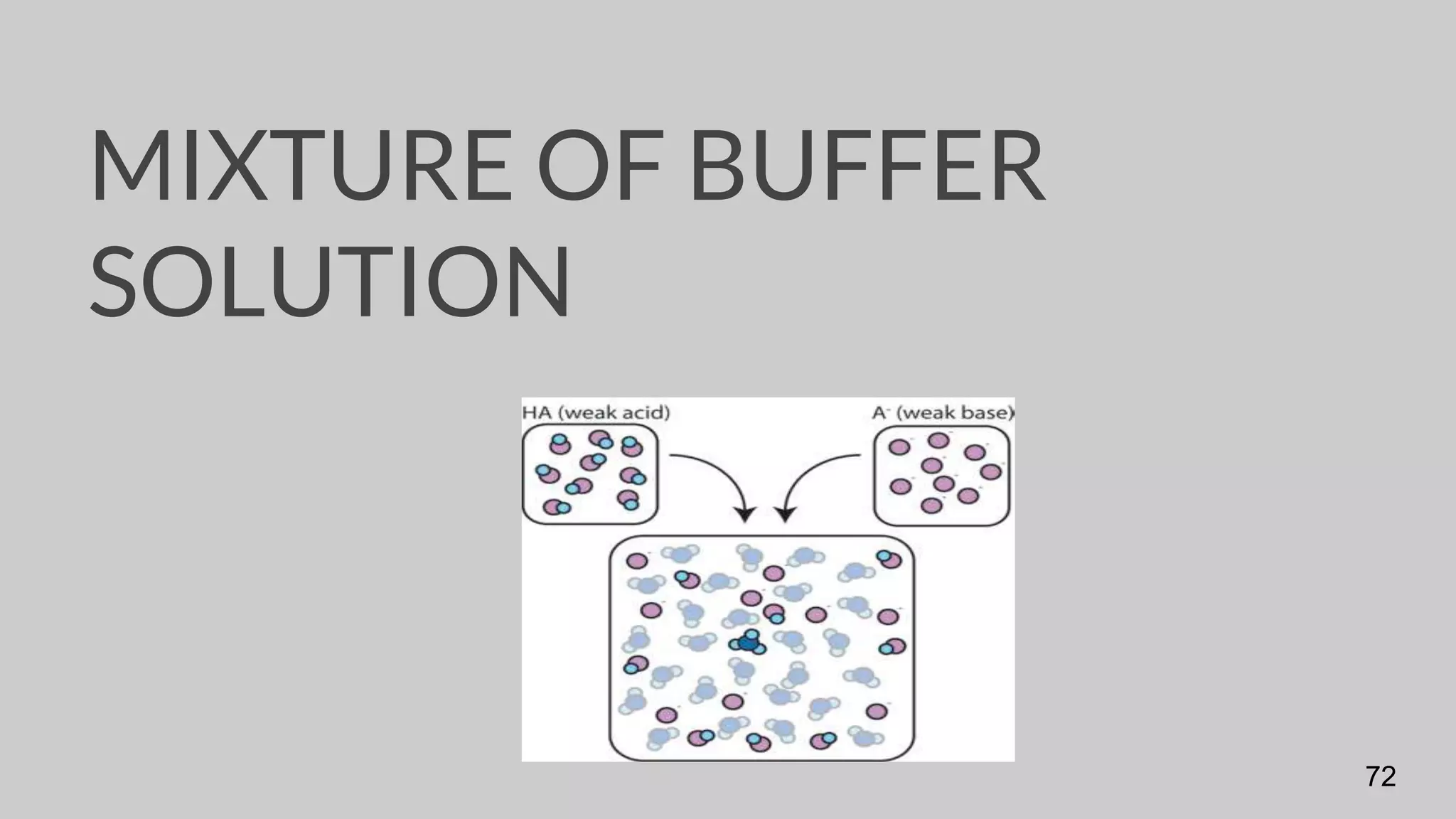
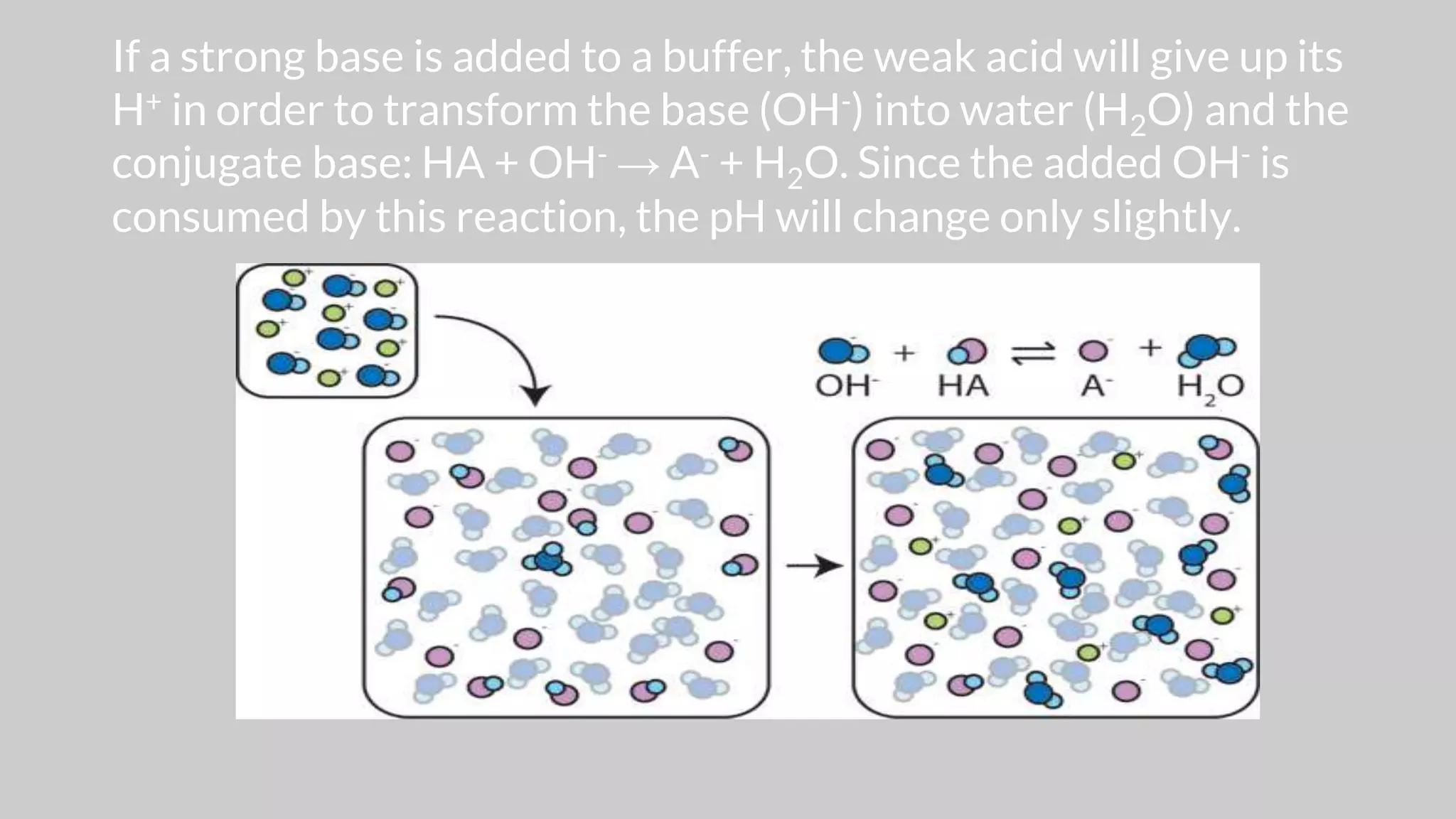
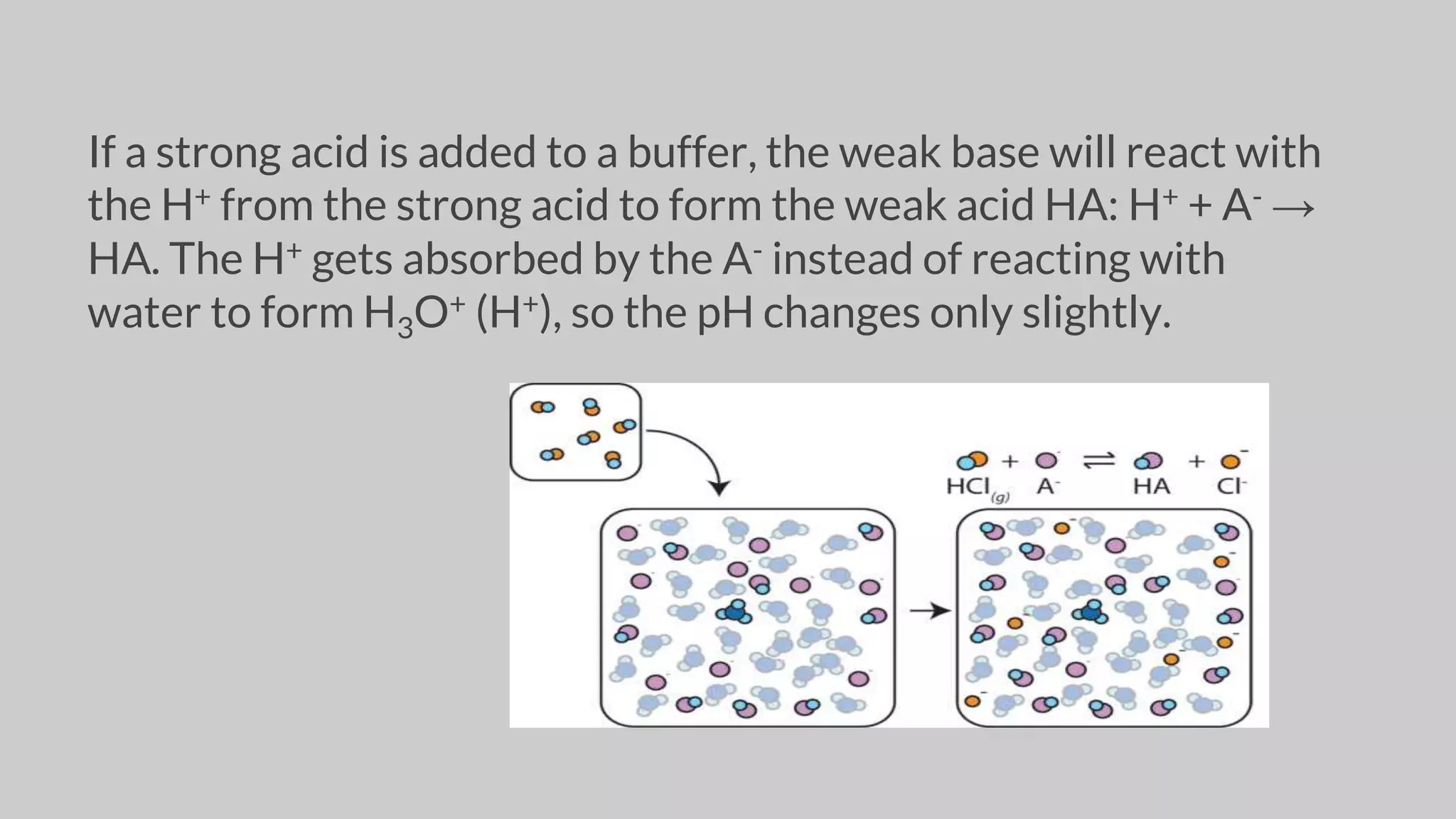
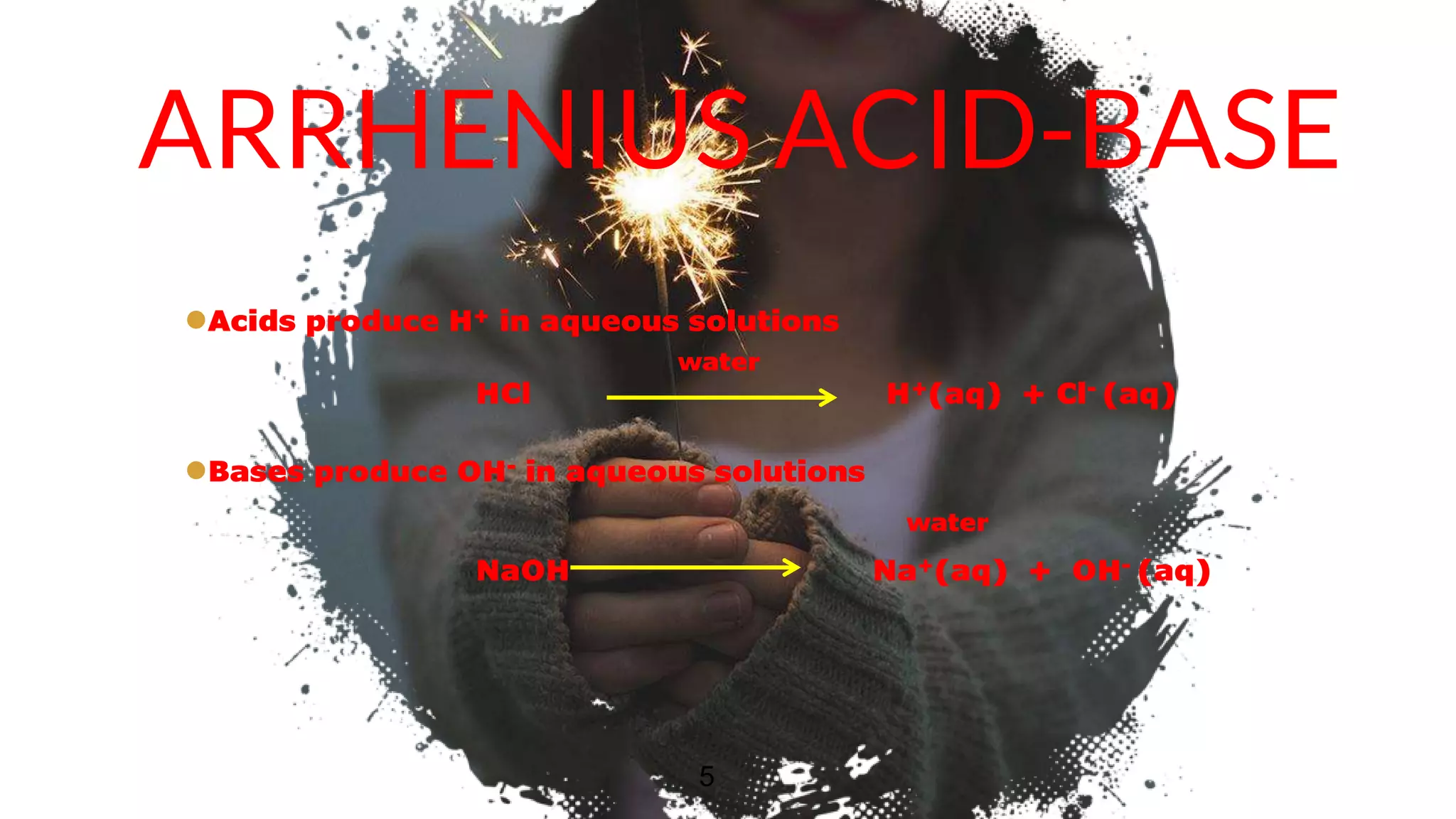
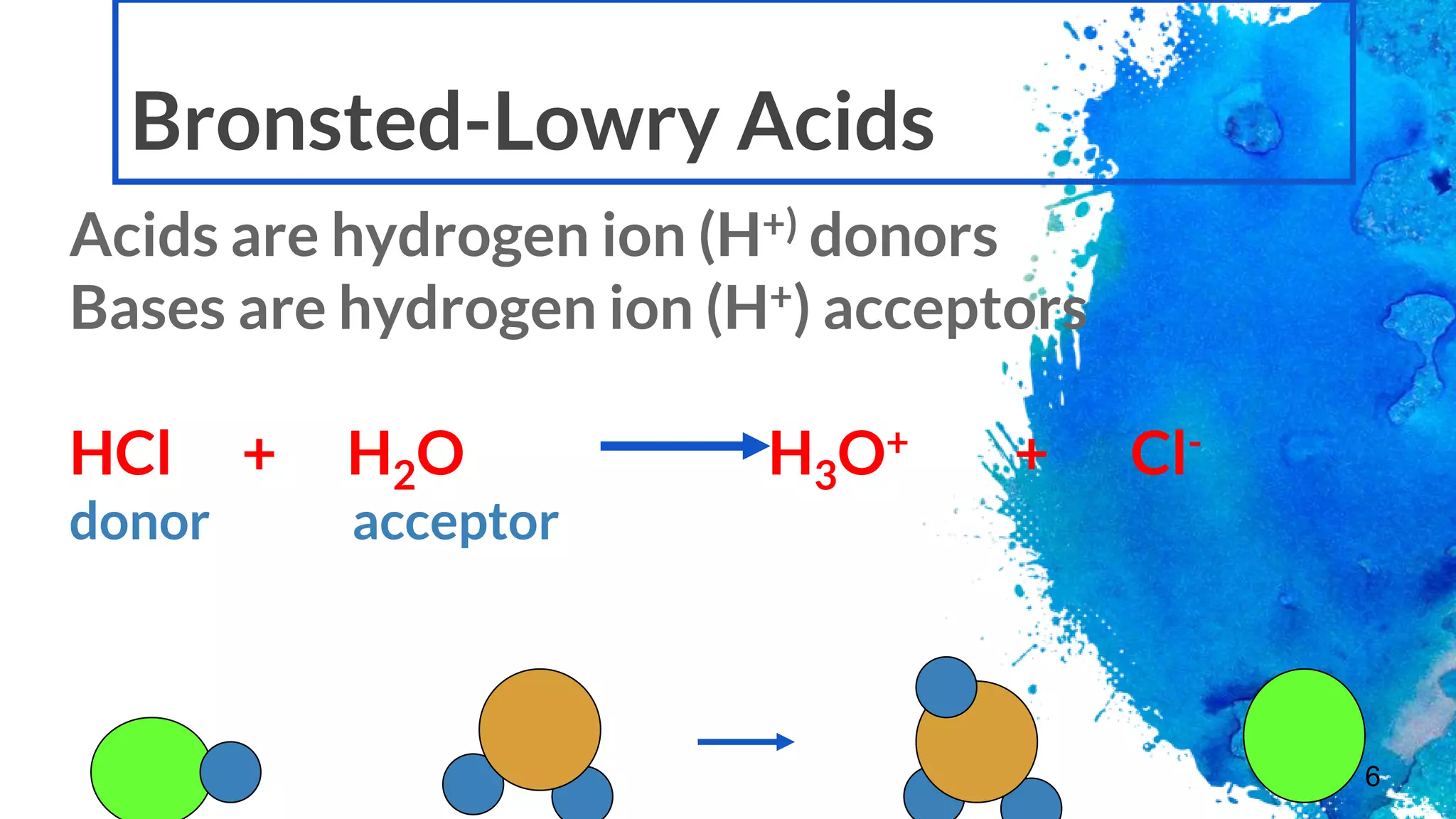
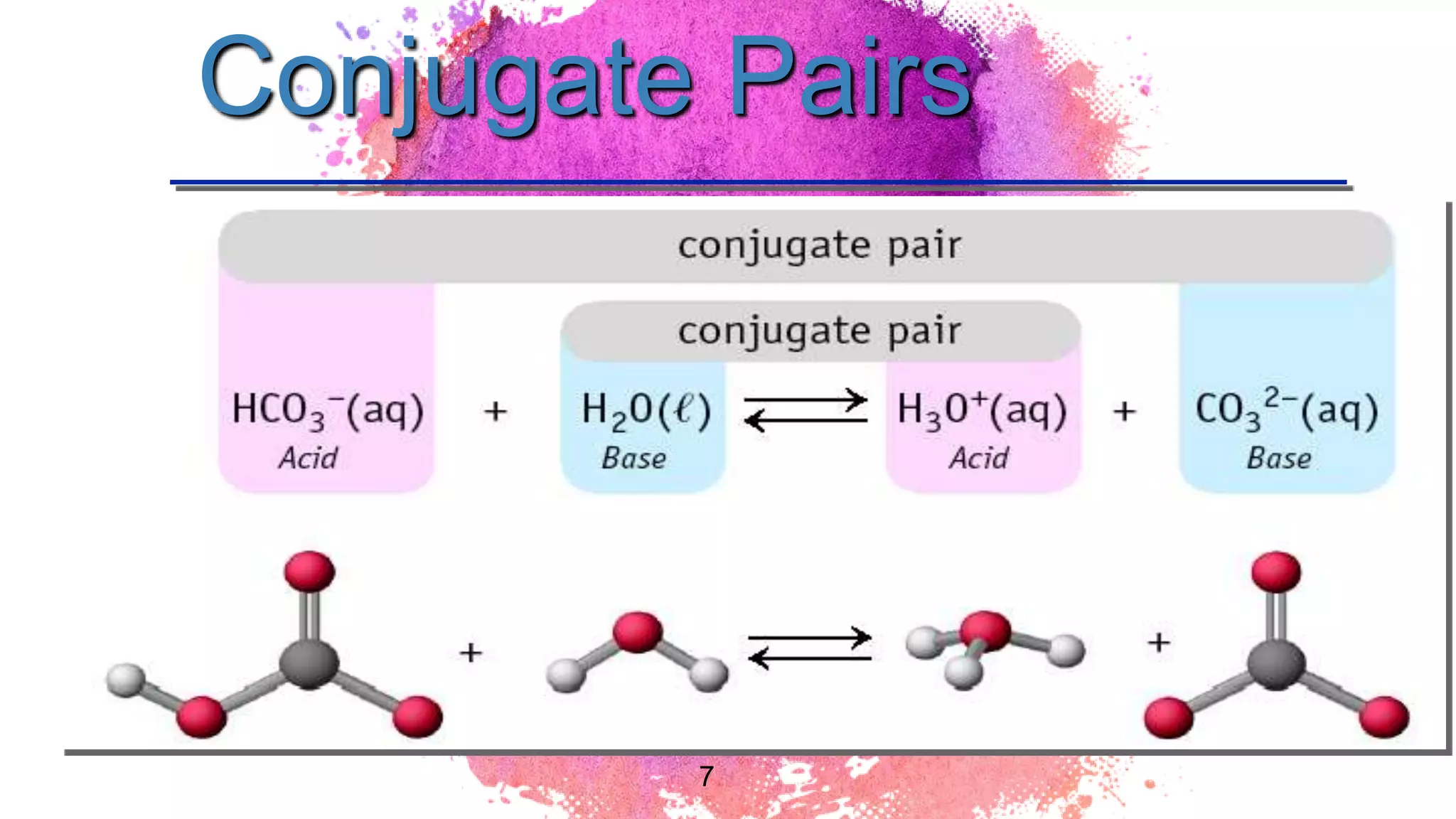
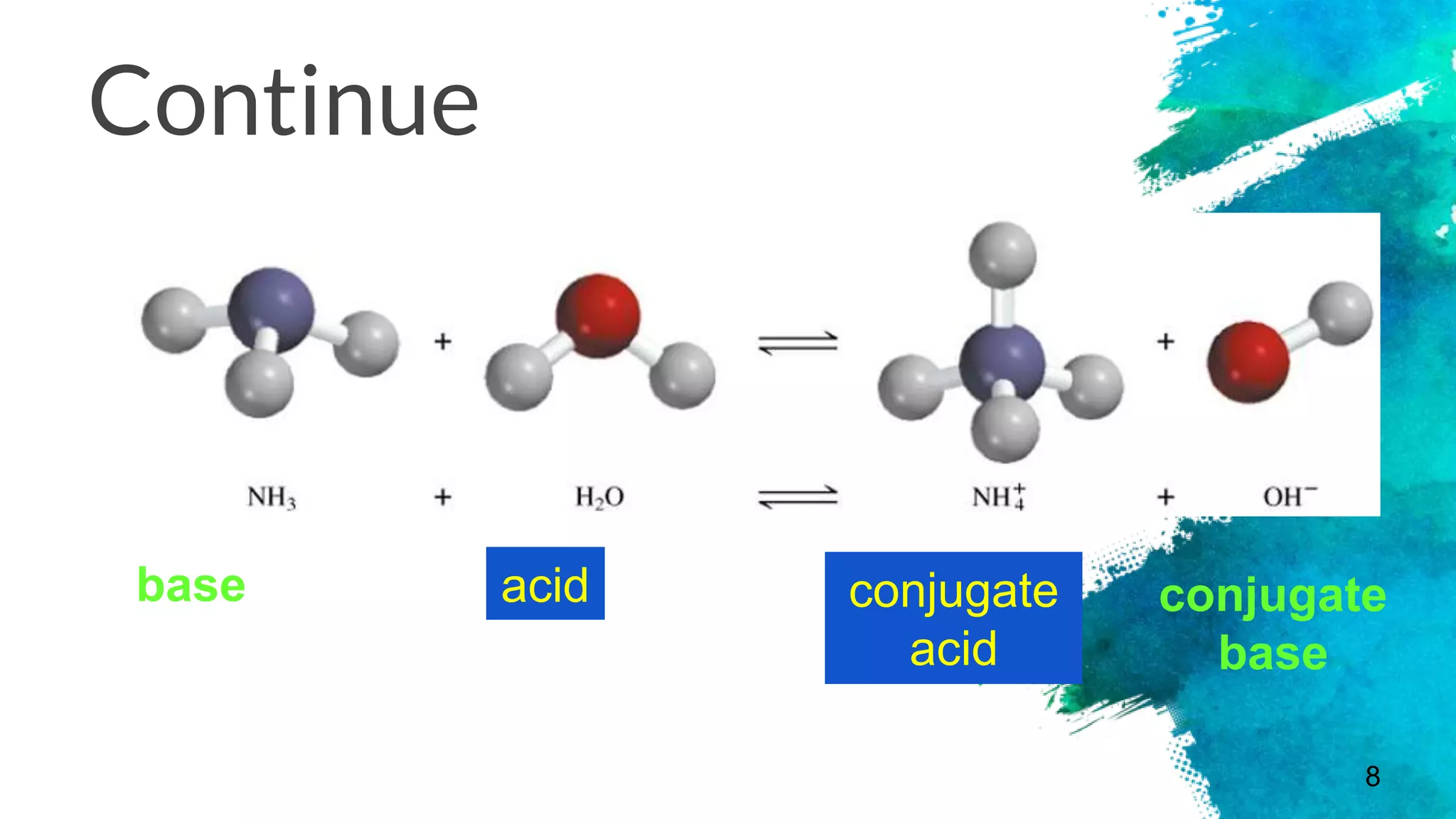
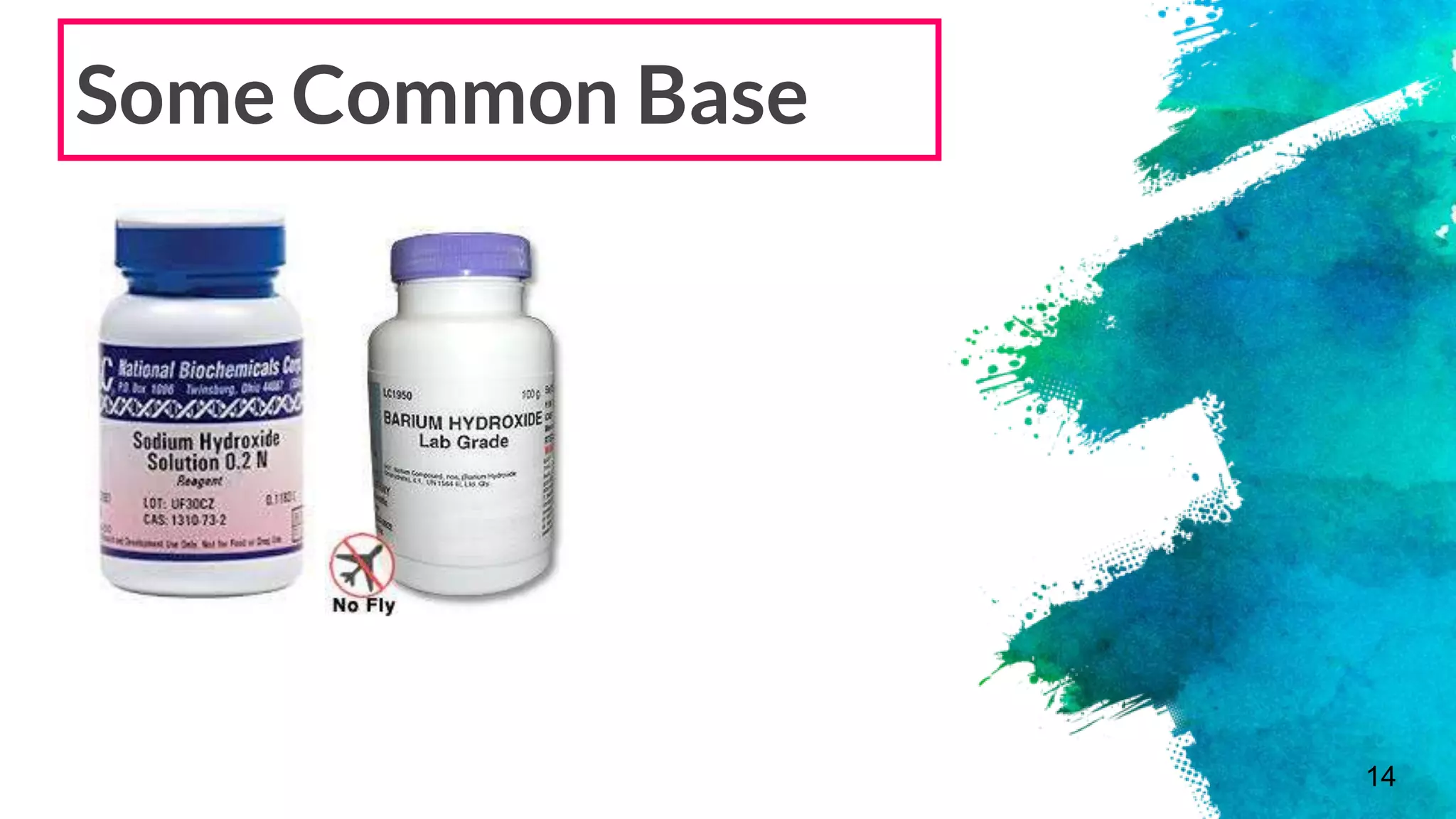
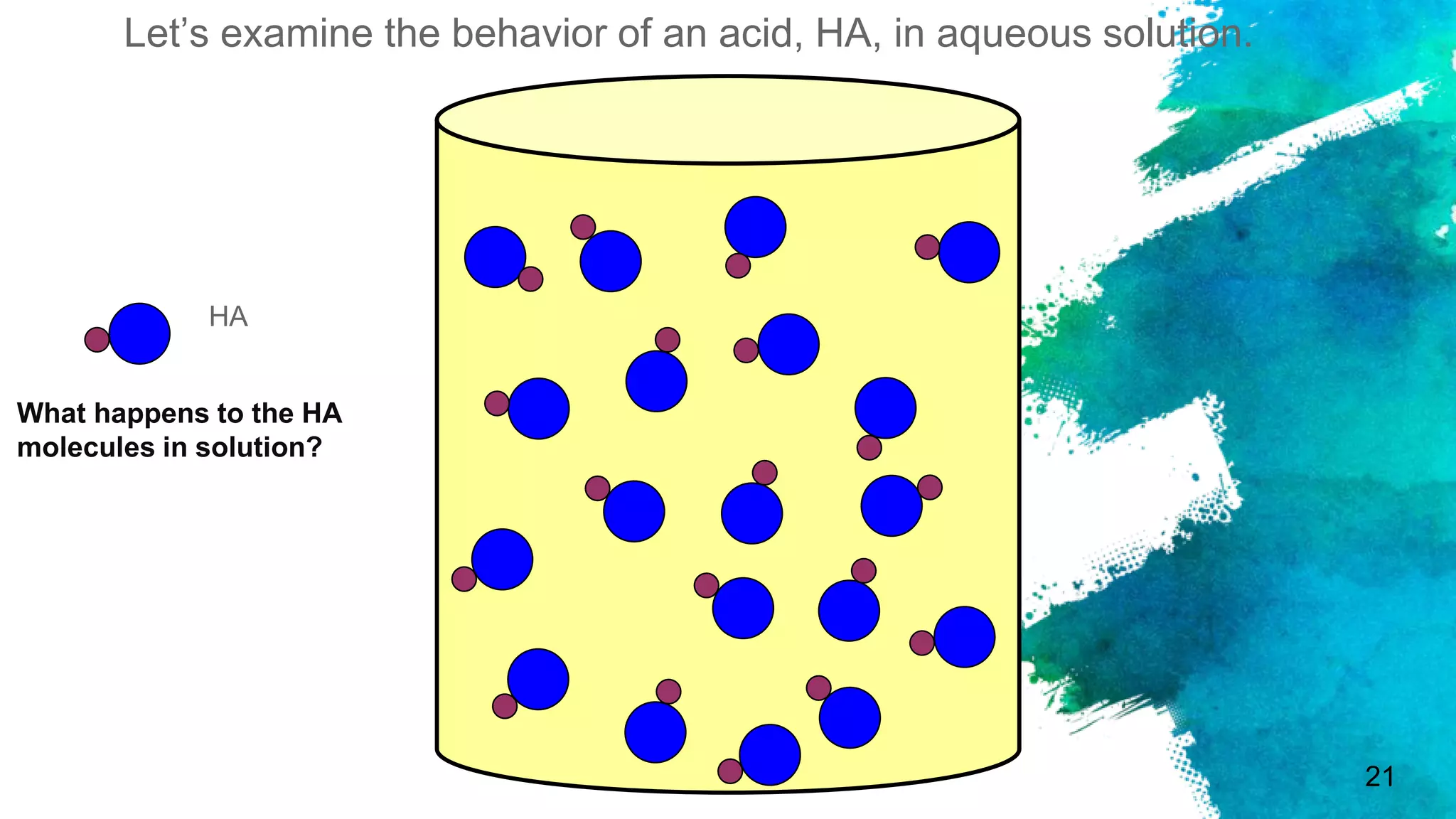
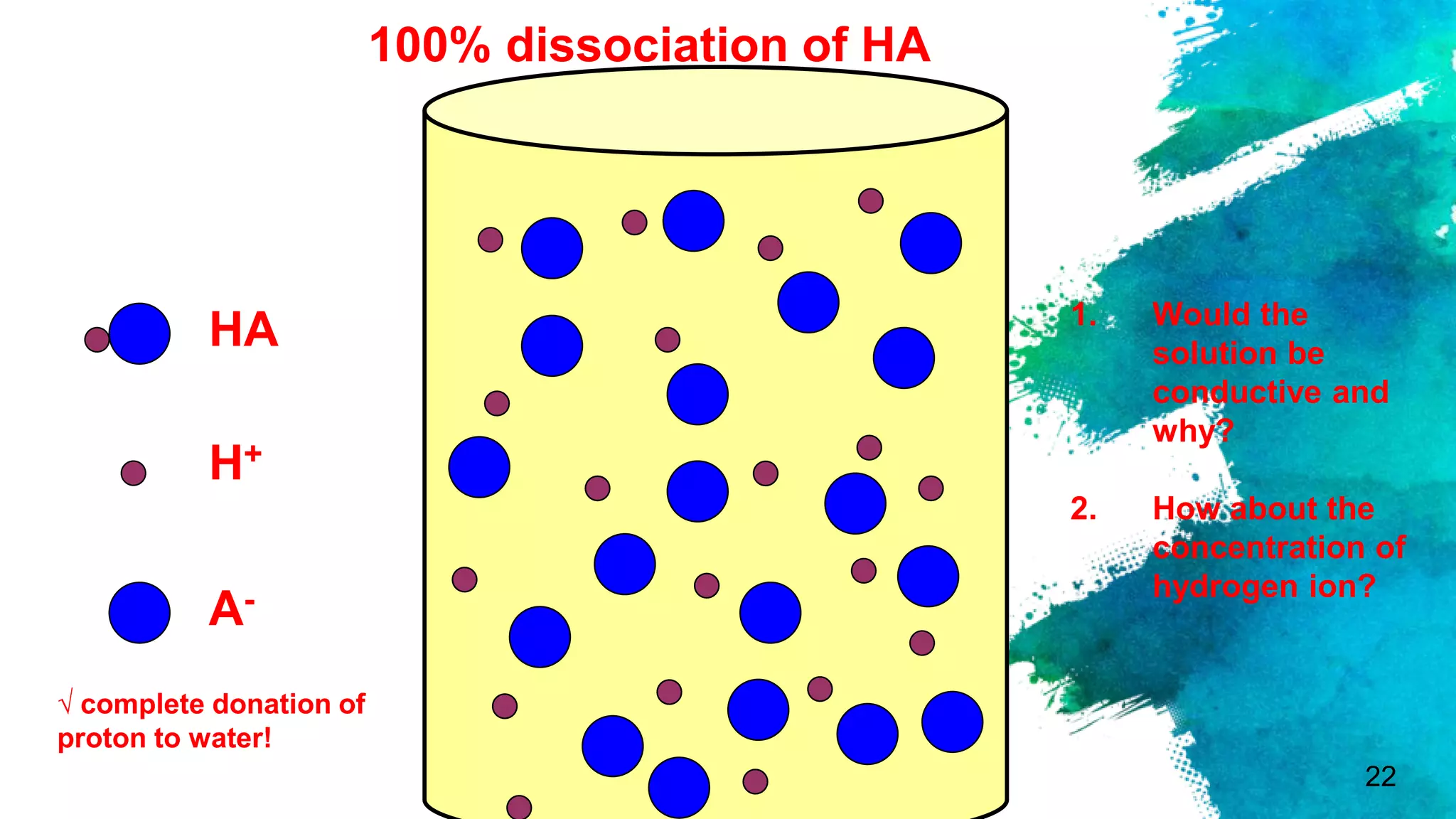
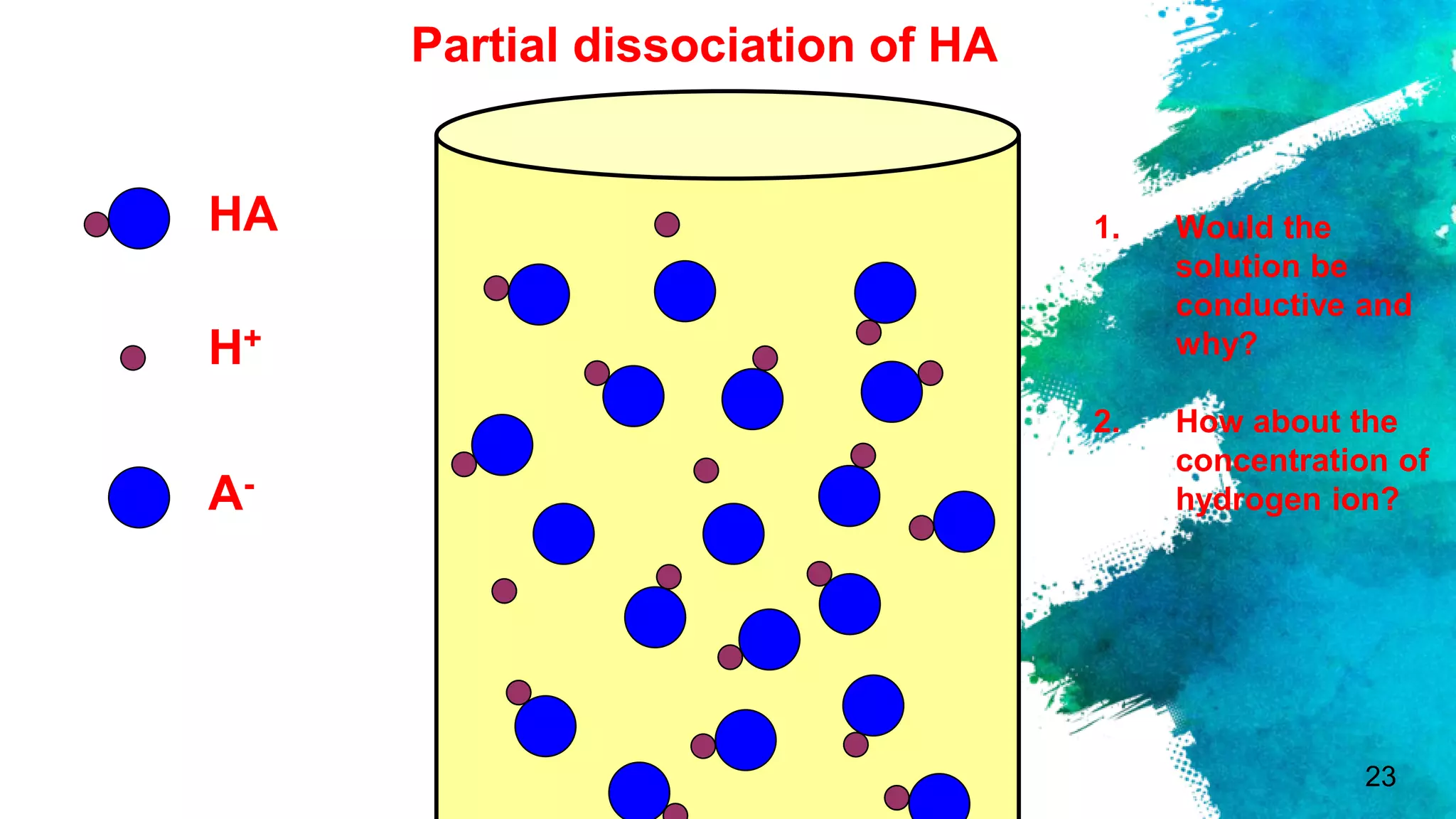
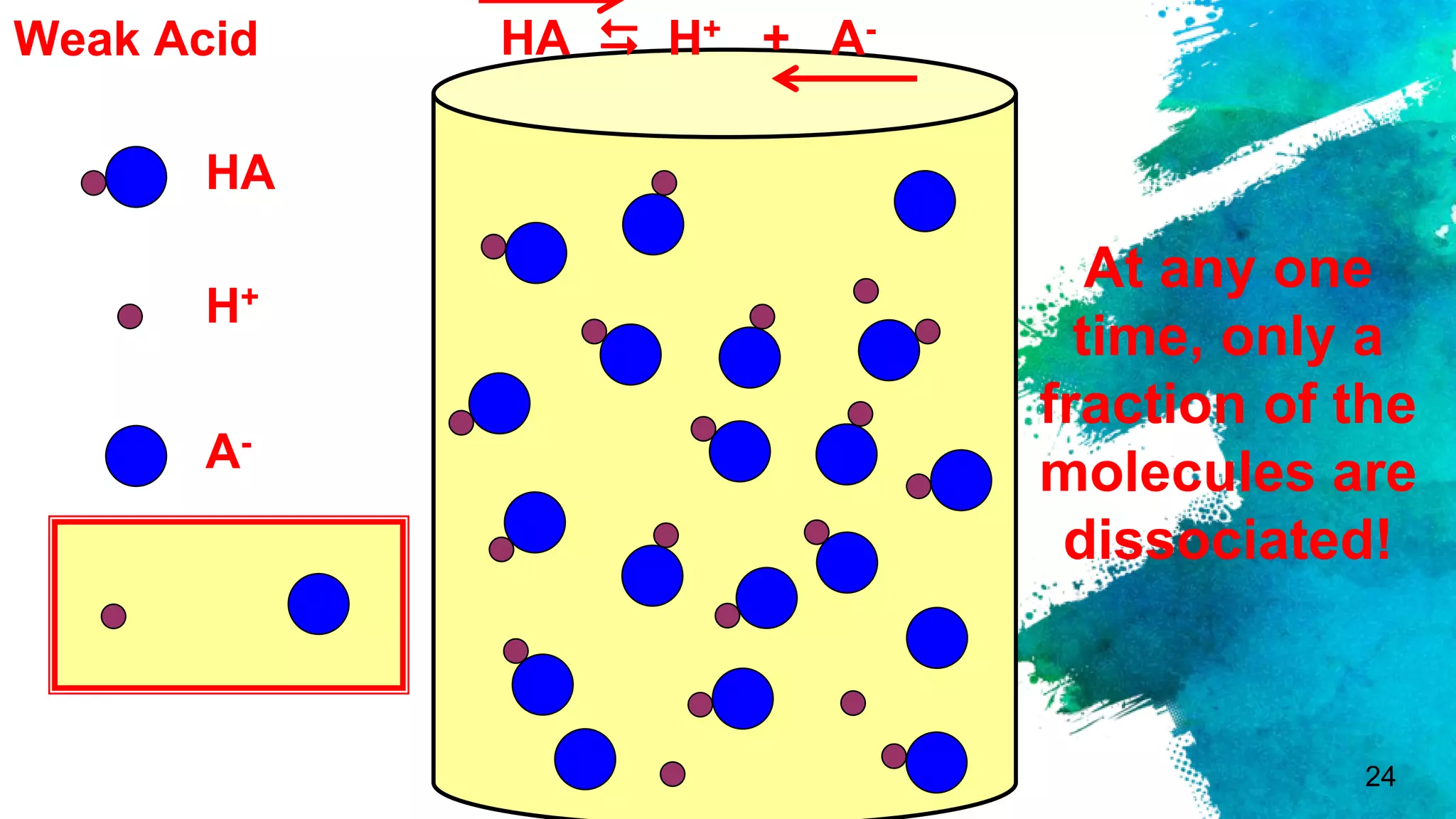
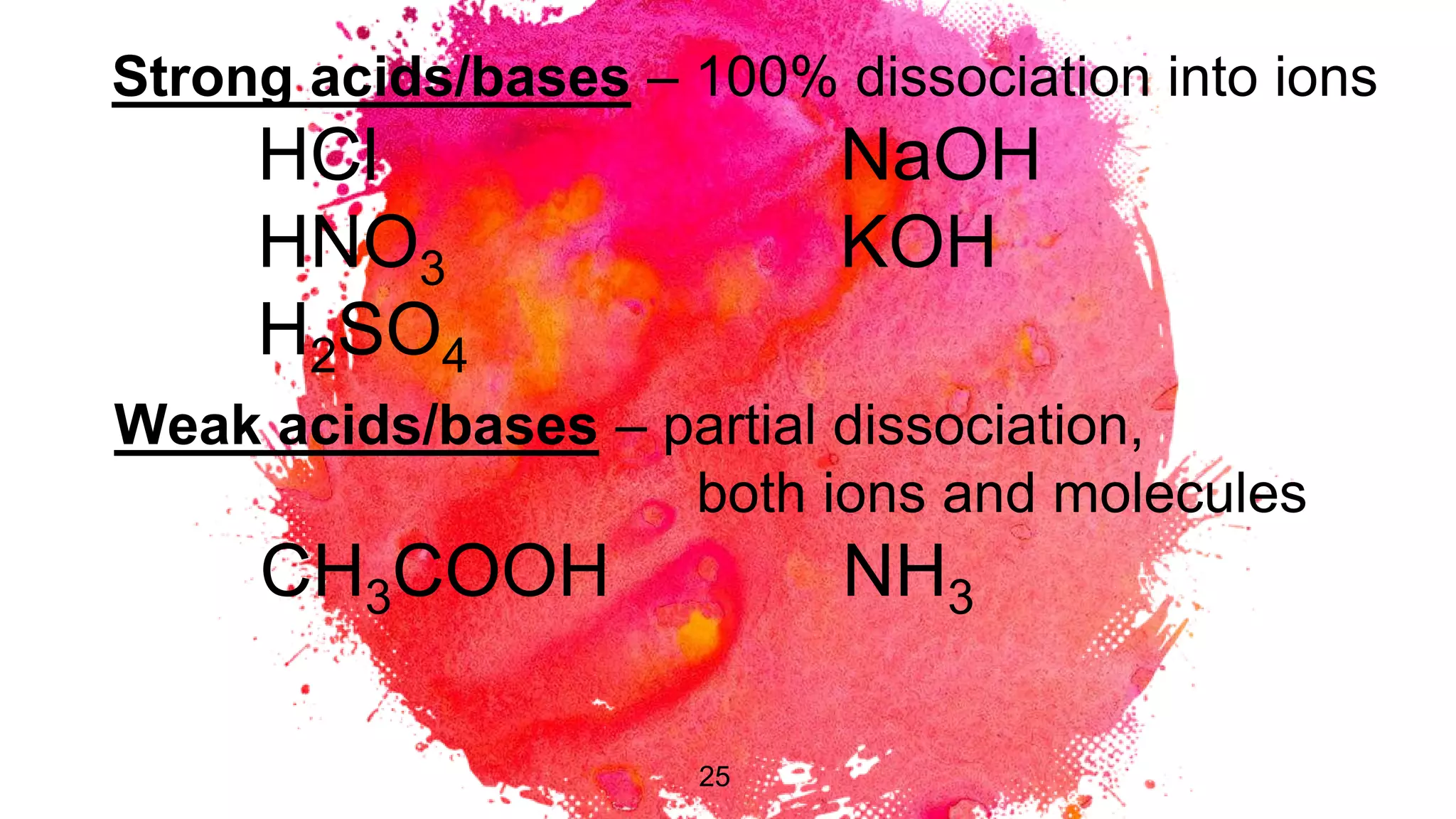
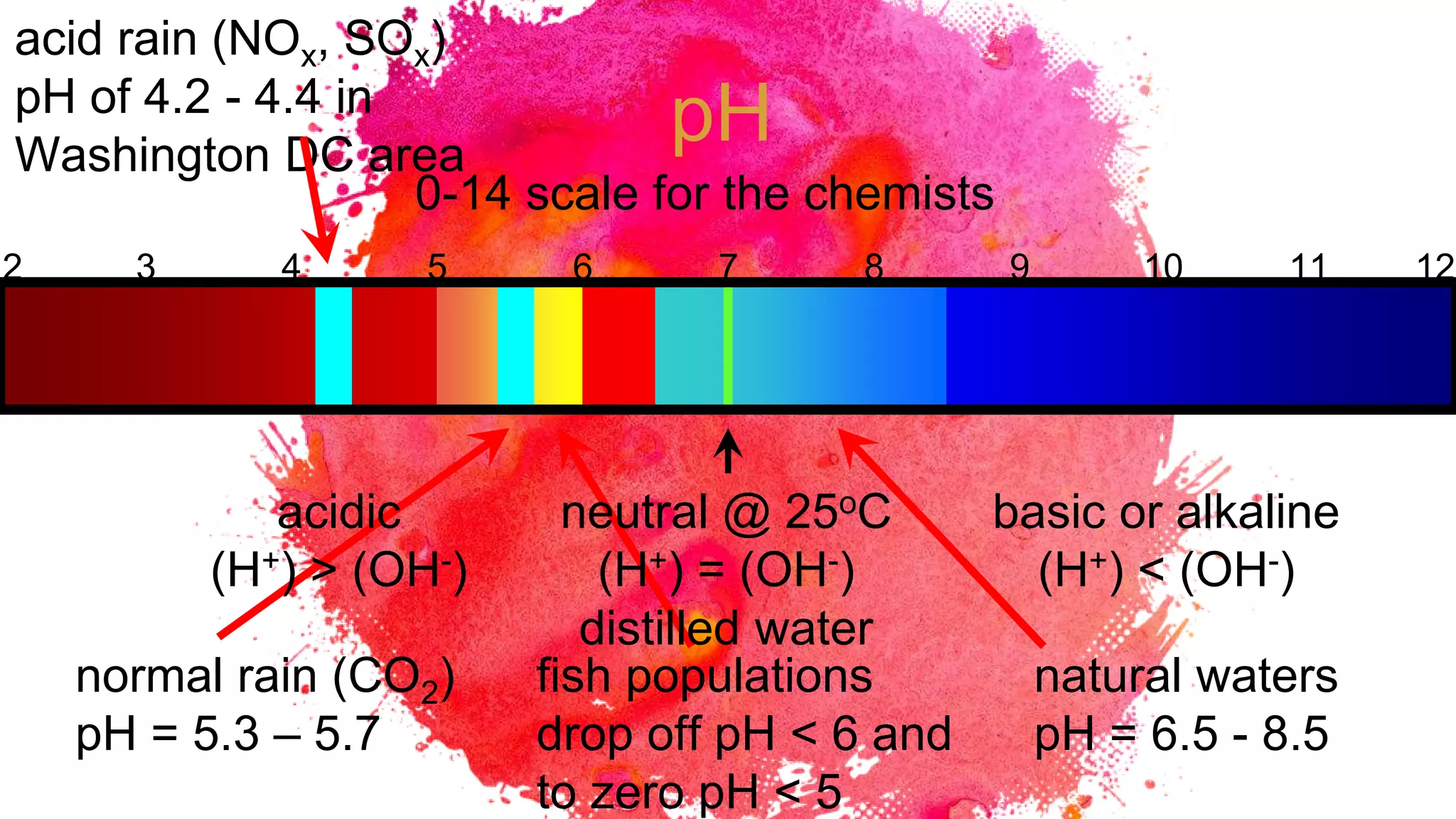
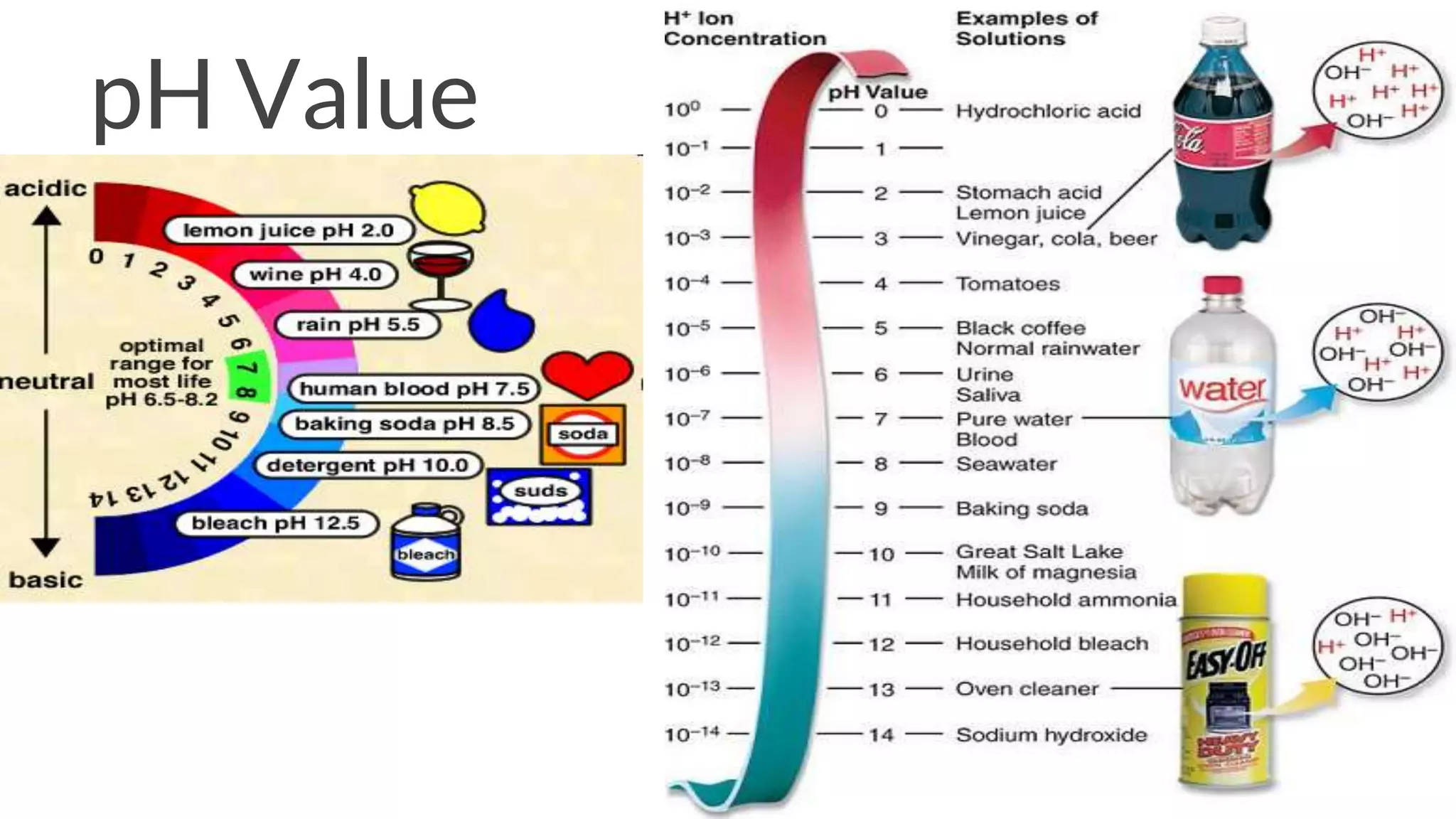
The document discusses acids and bases according to various theories including Arrhenius and Bronsted-Lowry. It defines acids as hydrogen ion donors and bases as hydrogen ion acceptors. Acids are classified as strong or weak based on their degree of ionization in water. Buffer solutions are introduced as mixtures that minimize pH changes from the addition of small amounts of acid or base. Common examples of acidic and alkaline buffer solutions are provided.









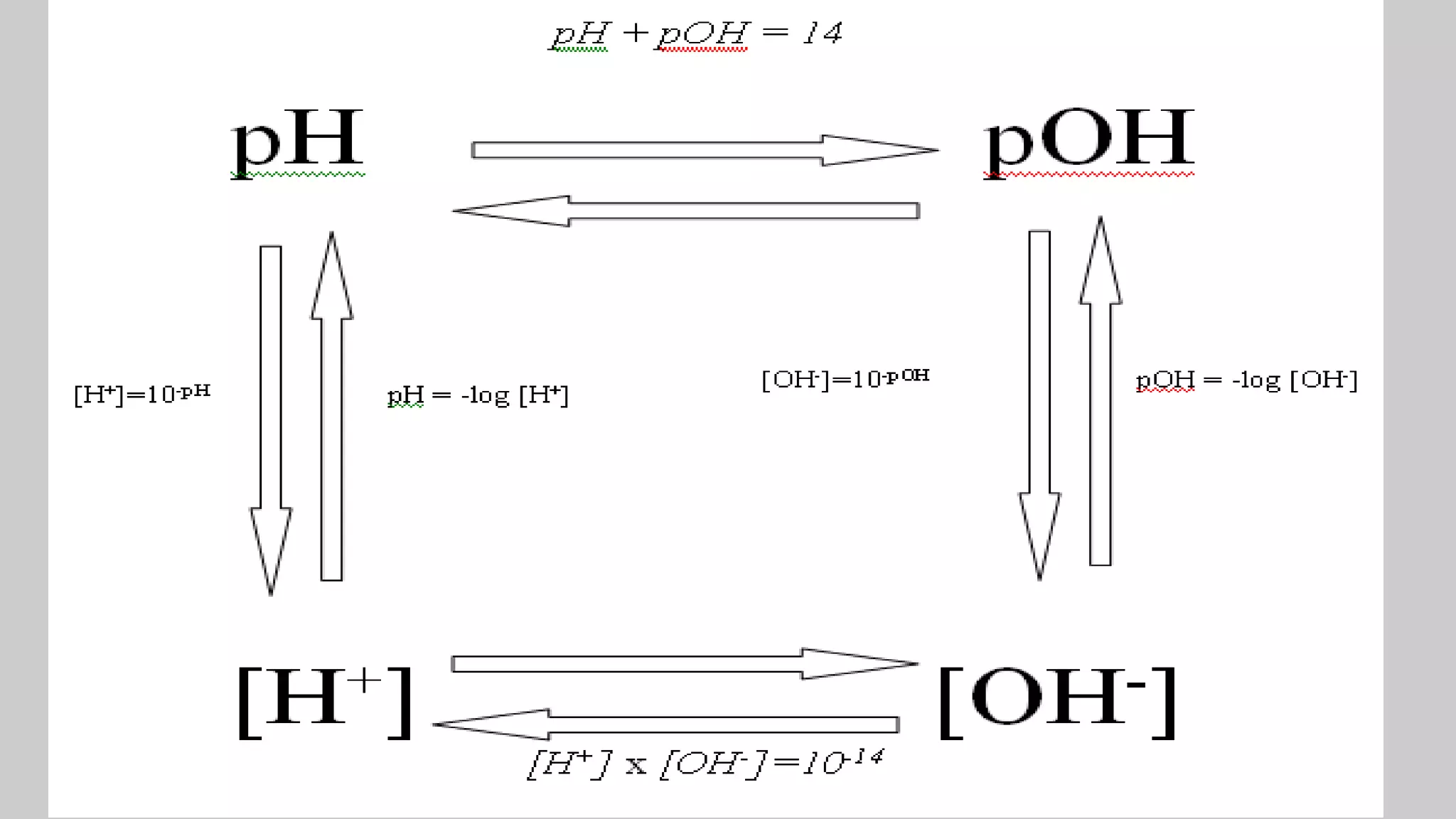
![× pH
The negative of the common logarithm of the hydrogen ion concentration
pH = - log [H+]
× pOH
The negative of the common logarithm of the hydroxide ion concentration
pOH = - log [OH-]
Calculating pH & pOH](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-28-2048.jpg)
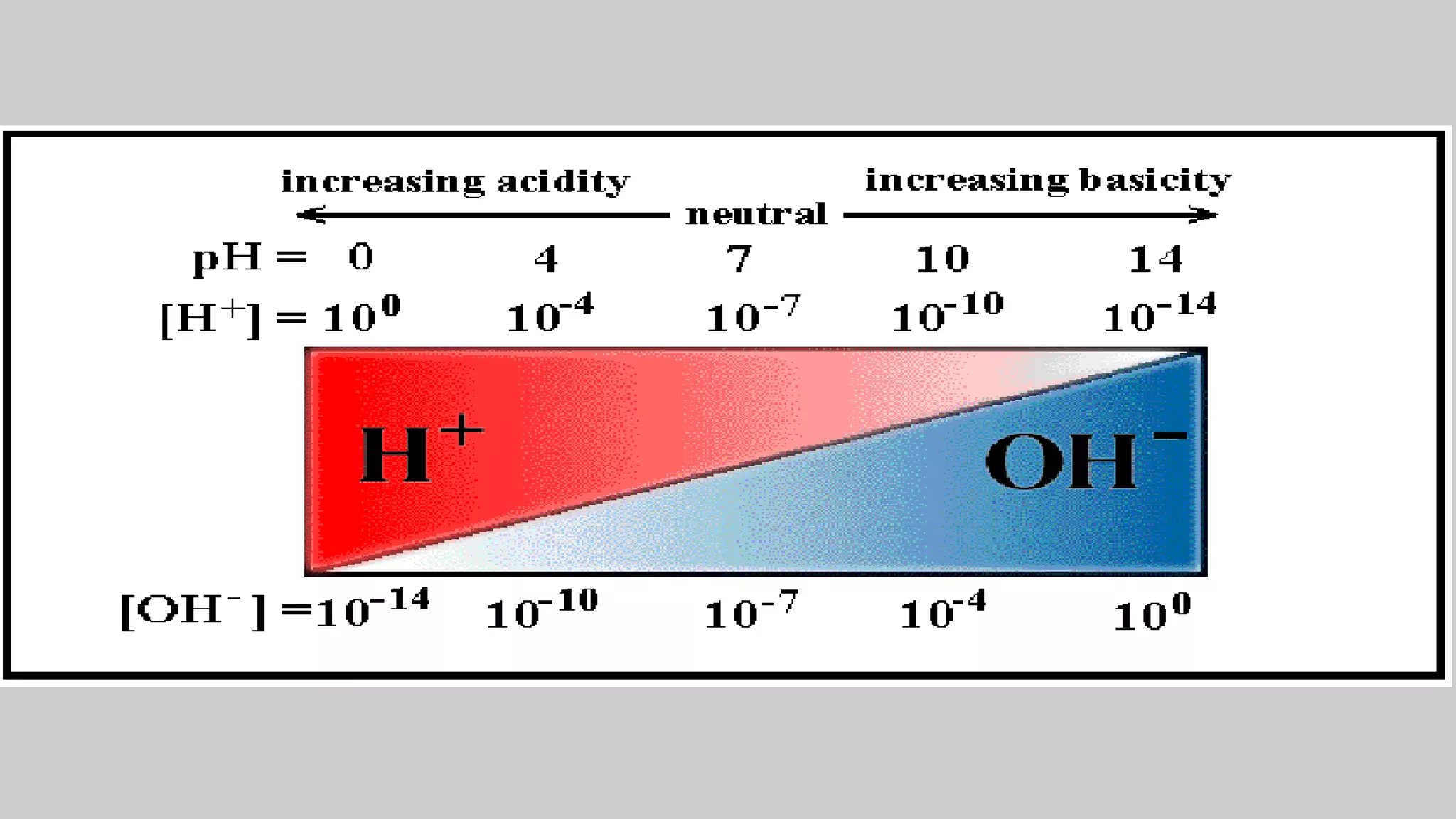
![× For acids
× For bases
× when [H+] = [OH-] the substance is neutral
Water breaks apart to hydrogen and hydroxide ions:
× H2O H+ + OH-
× pH + pOH = 14
H+1 > OH-1
OH-1 > H+1](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-29-2048.jpg)
![More About Water
H2O can function as both an ACID and a BASE.
In pure water there can be AUTOIONIZATION
Equilibrium constant for water = Kw
K = [H O+] [OH-] = 1.00 x 10-14 at 25 oC](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-30-2048.jpg)
![Kw = [H3O+] [OH-] = 1.00 x 10-14 at 25 oC
In a neutral solution [H3O+] = [OH-]
and so [H3O+] = [OH-] = 1.00 x 10-7 M
More About Water
OH-
H3O+
Autoionization](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-31-2048.jpg)




![If the pH of Coke is 3.12, [H+] = ???
Because pH = - log [H+] then
- pH = log [H+]
Take antilog (10x) of both
sides and get
10-pH = [H+]
[H+] = 10-3.12 = 7.6 x 10-4 M
*** to find antilog on your calculator, look for “Shift” or “2nd function” and then
the log button
pH calculations – Solving for H+](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-38-2048.jpg)
![What is the pH of a 2 x 10-3 M HNO3 solution?
HNO3 is a strong acid – 100% dissociation.
HNO3 (aq) + H2O (l) H3O+ (aq) + NO3
- (aq)
pH = -log [H+] = -log [H3O+] = -log(0.002) = 2.7
Start
End
0.002 M
0.002 M 0.002 M
0.0 M
0.0 M 0.0 M
What is the pH of a 1.8 x 10-2 M Ba(OH)2 solution?
Ba(OH)2 is a strong base – 100% dissociation.
Ba(OH)2 (s) Ba2+ (aq) + 2OH- (aq)
Start
End
0.018 M
0.018 M 0.036 M
0.0 M
0.0 M 0.0 M
pH = 14.00 – pOH = 14.00 + log(0.036) = 12.56
15.4](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-40-2048.jpg)

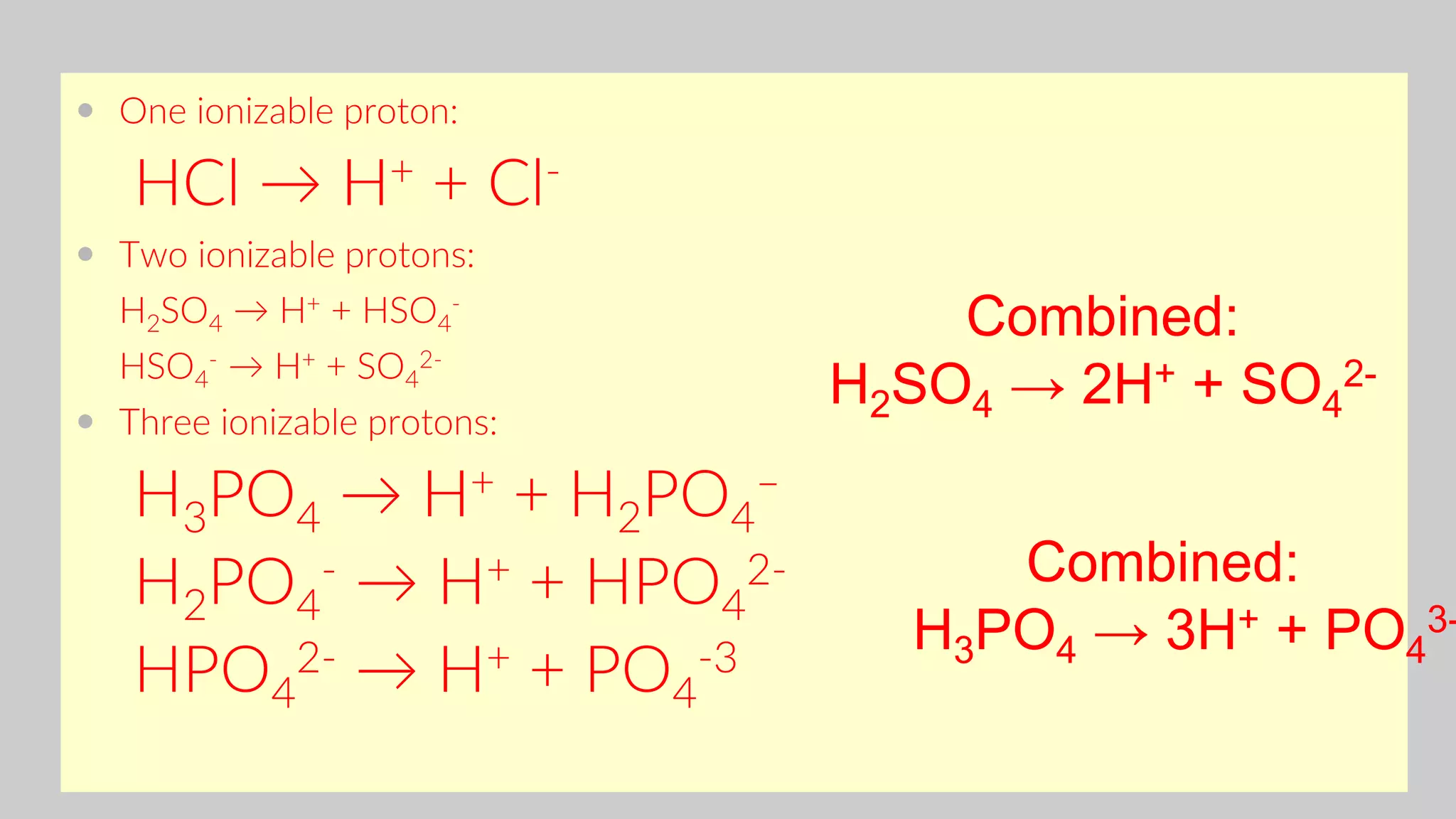



![Consider acetic acid, HC2H3O2 (HOAc)
HC2H3O2 + H2O ↔ H3O+ + C2H3O2
-
Acid Conj. base
Equilibria Involving
Weak Acids and Bases
Ka
[H3O+][OAc- ]
[HOAc]
1.8 x 10-5
(K is designated Ka for ACID)
K gives the ratio of ions (split up) to molecules (don’t split up)](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-46-2048.jpg)
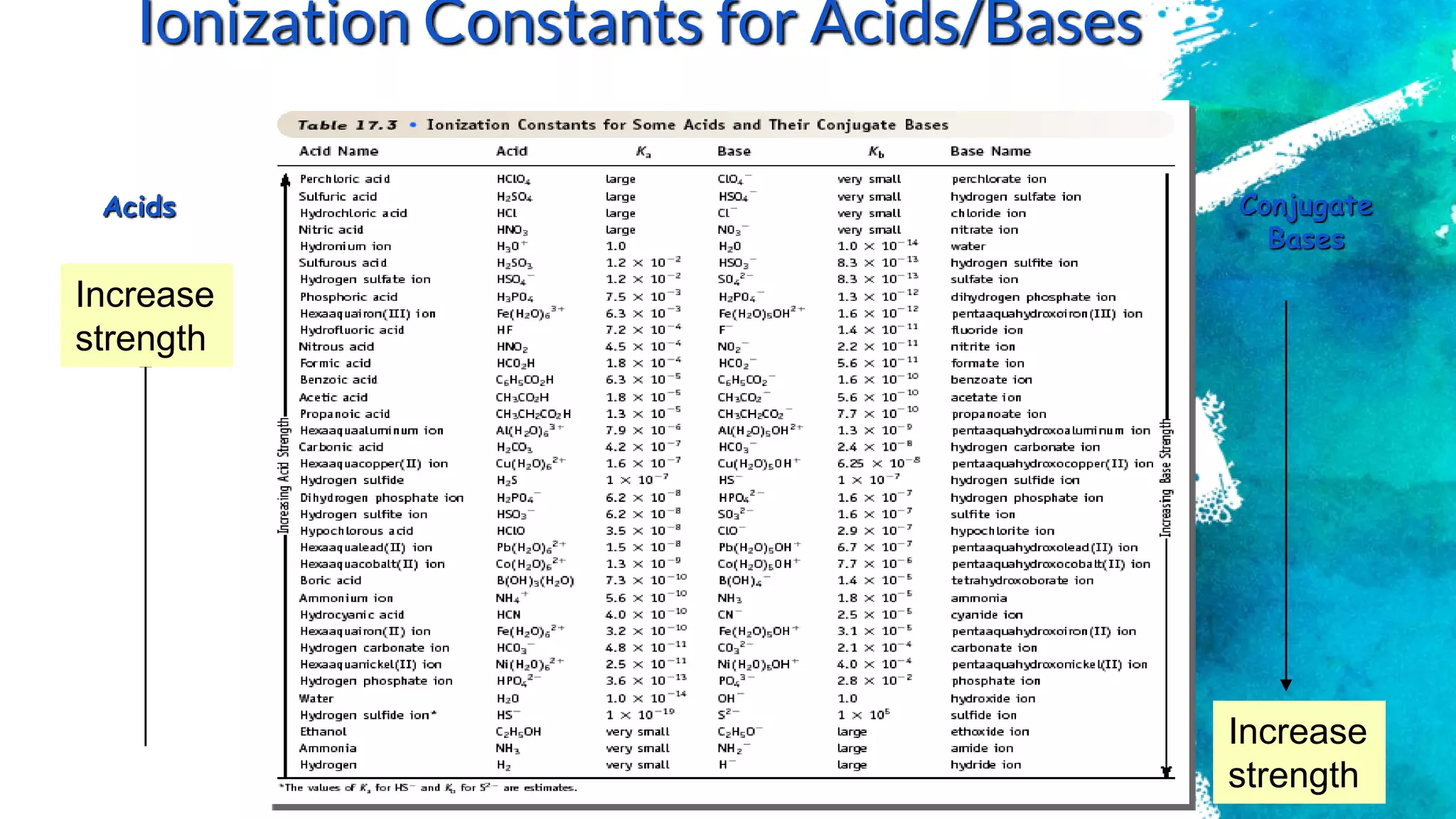
![Equilibrium Constants
for Weak Acids
Weak acid has Ka < 1
Leads to small [H3O+] and a pH of 2 - 7](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-48-2048.jpg)
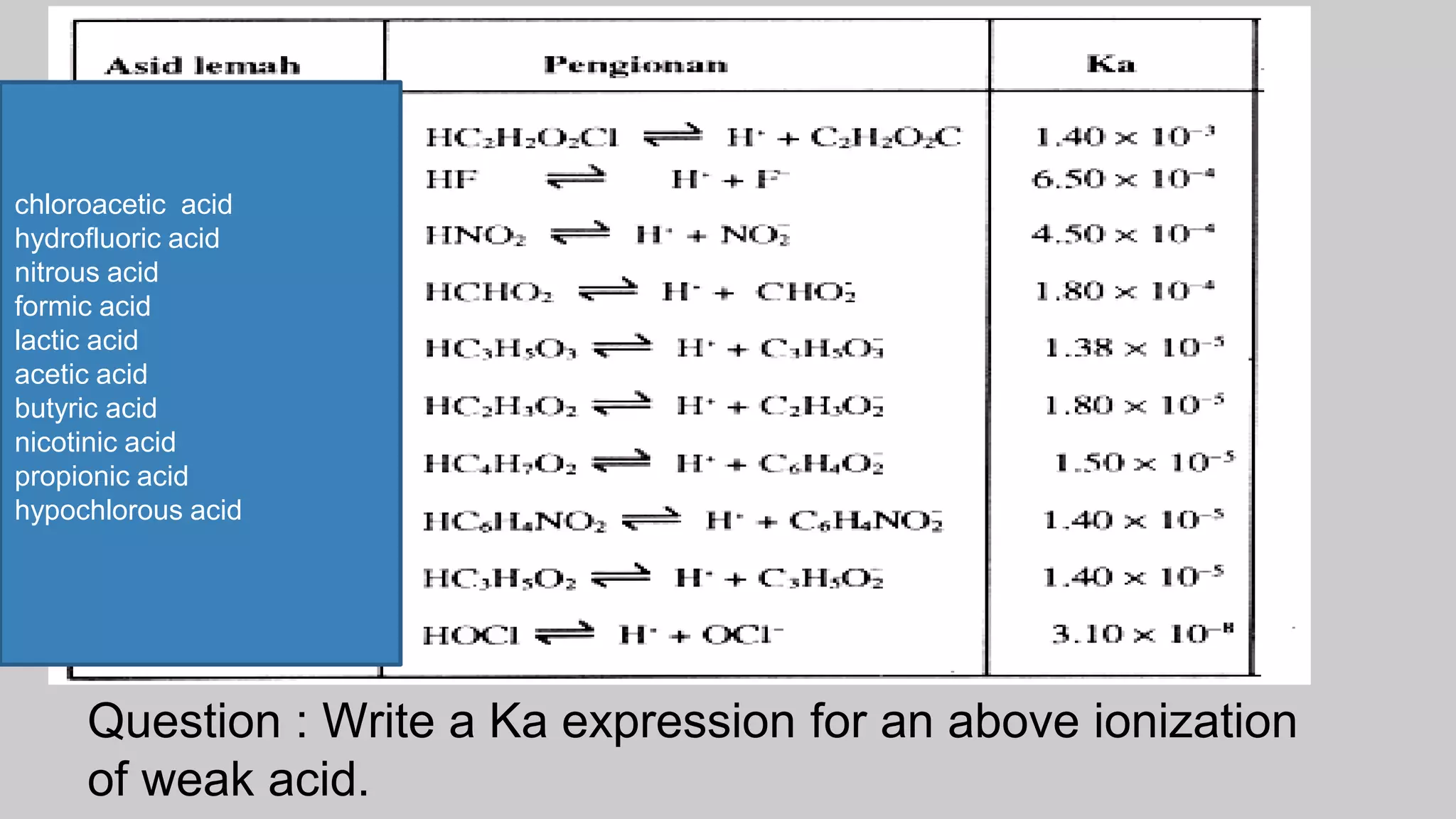

![ Solution
Dissociation of caproic acid is shown below:
HCap(aq) H+ + Cap-(aq)
Initial Concentration : 0.030 M 0 M 0 M
Concentration at equilibrium : 0.030 – x) M x M x M
At equilibrium
Ka = [H+][Cap-]
[HCap]
[H+] = [Cap-]
= 6.5 x 10-4 M
therefore [HCap] =3.0 x 10-2 M - 6.5 x 10-4 M
= 2.9 x 10-2 M
Ka = (6.5 x 10-4) (6.5 x 10-4)
2.9 x 10-2
= 1.5 x 10-5](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-51-2048.jpg)
![Equilibrium Constants
for Weak Bases
Weak base has Kb < 1
Leads to small [OH-] and a pH of 12 - 7](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-52-2048.jpg)

![Calculate [OH-] in 0.20 M aqueous NH3. (Kb = 1.80 x 10-5)
Solution
NH3 + H20 NH4
+ + OH-
Initial Concentration : 0.2 M 0 M 0 M
Concentration at equilibrium: (0.2 – x ) M x M x M
At equilibrium
Kb = [NH4
+][OH-]
[NH3]
= 1.80 x 10-5
= x2 .
0.20 – x
Kb value is very small, as a result 0.20 – x 0.2,
x2 = (1.8 x 10-5)(0.2)
x = [OH-]
= 1.9 X 10–3 M](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-54-2048.jpg)
![It can be seen that the calculation for weak acid and base is long and
complicated. There is a simple way to calculate that is by using the following
formula.
For weak acid:
[ H+] =
For weak base:
[ OH-] =
note: c represents the concentration of the acid or base](https://image.slidesharecdn.com/chapter1acidandbasessesi2-220315040203/75/Chapter-1-acid-and-bases-sesi-2-55-2048.jpg)