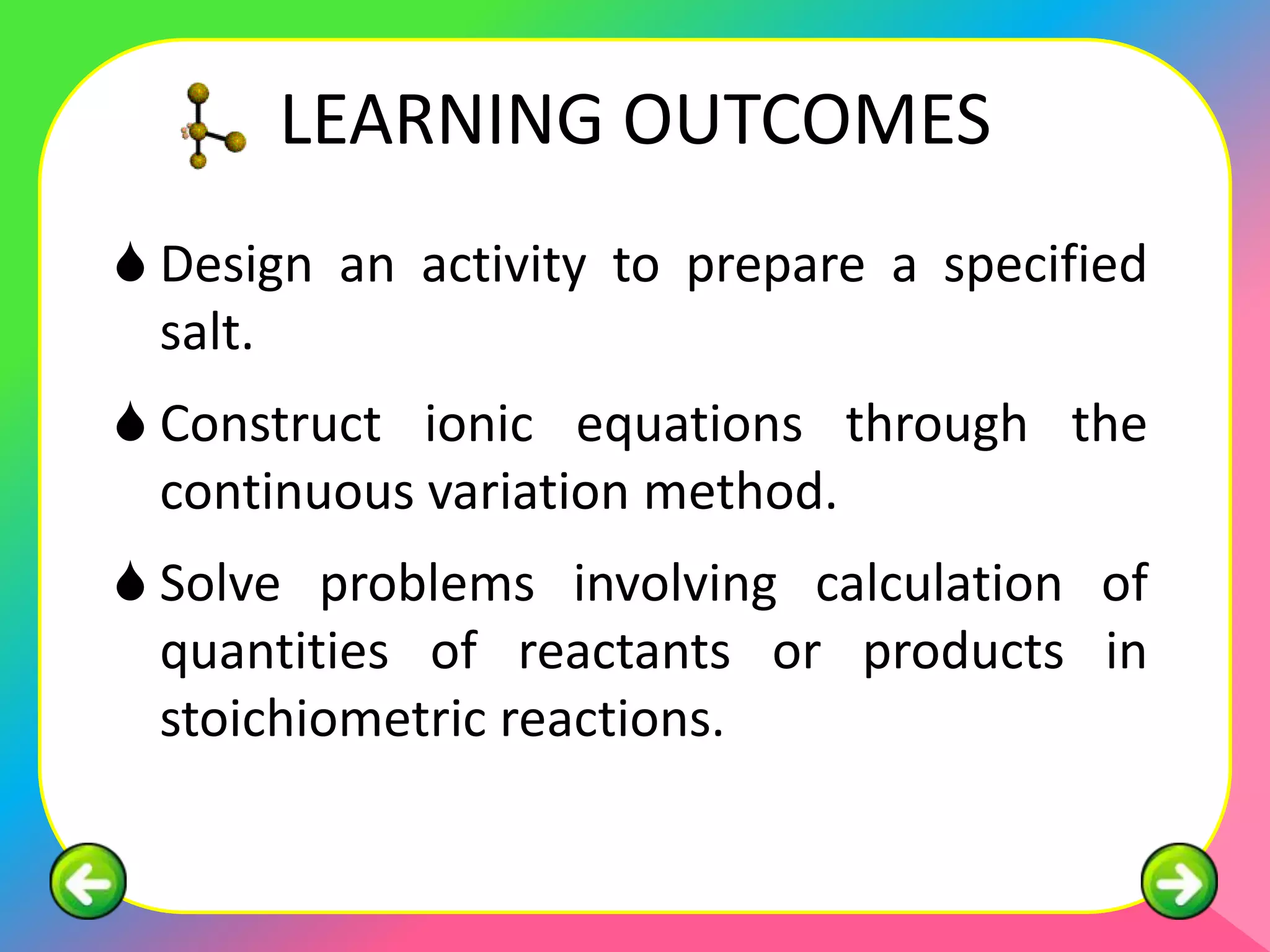
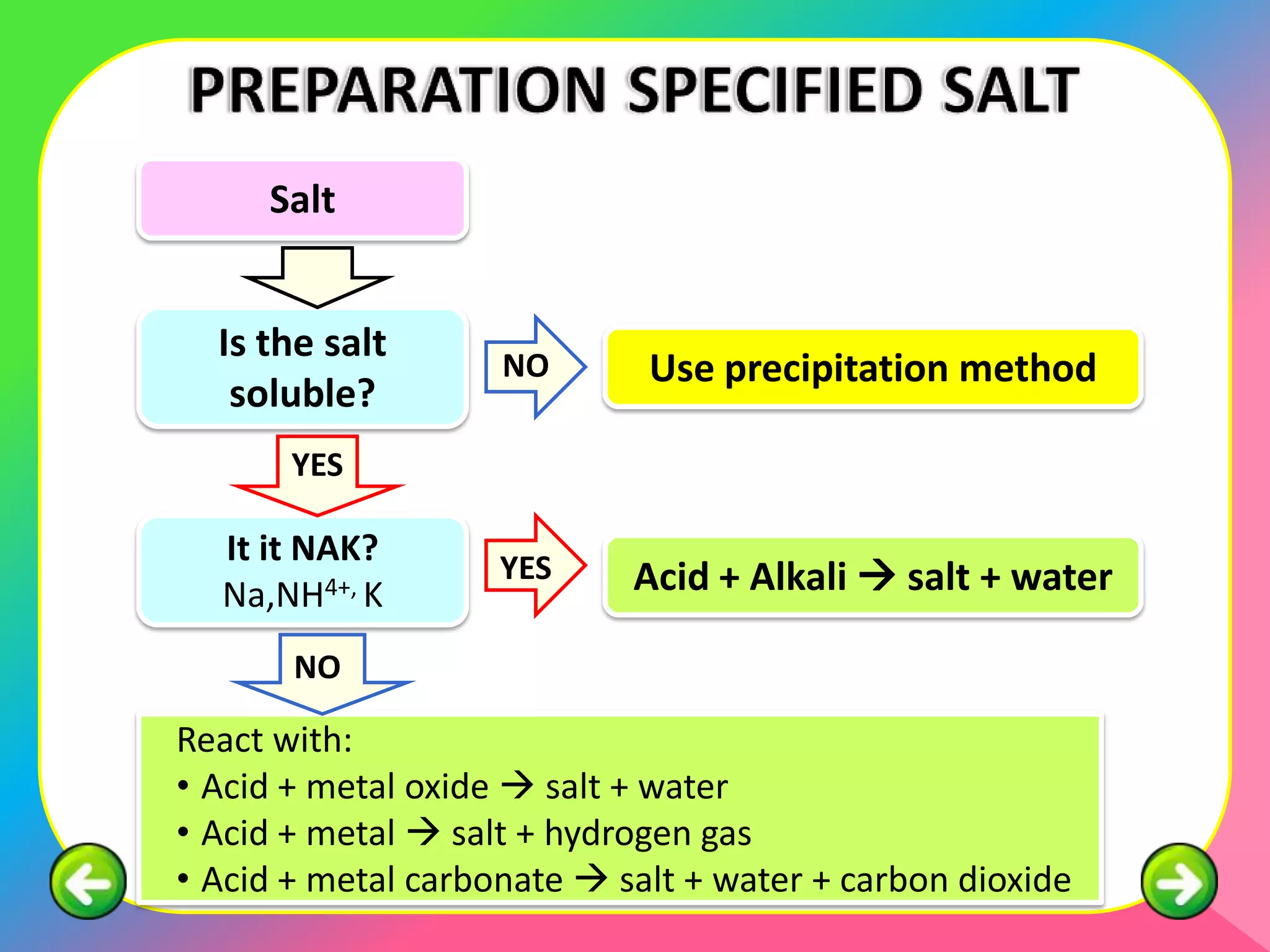
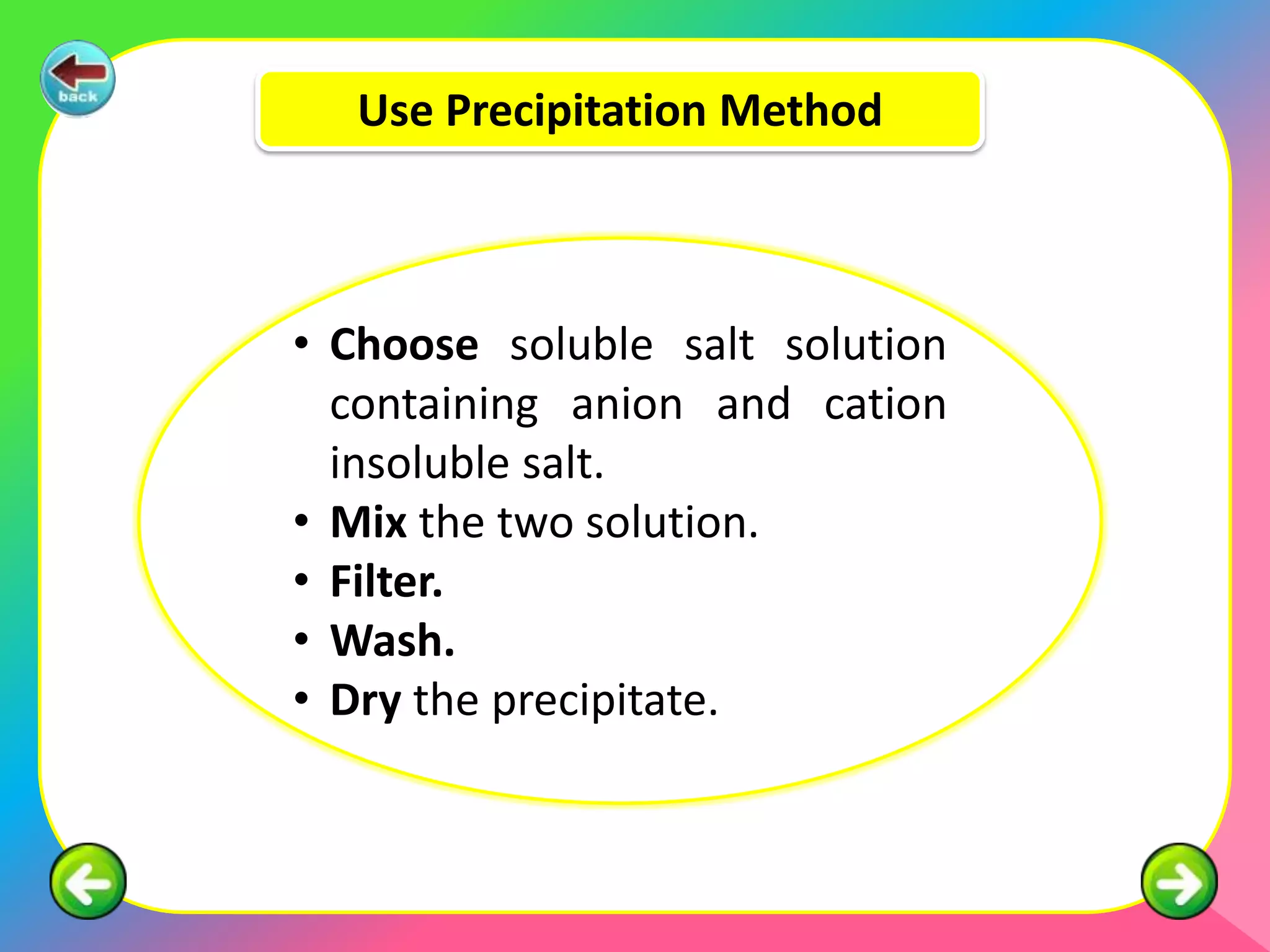
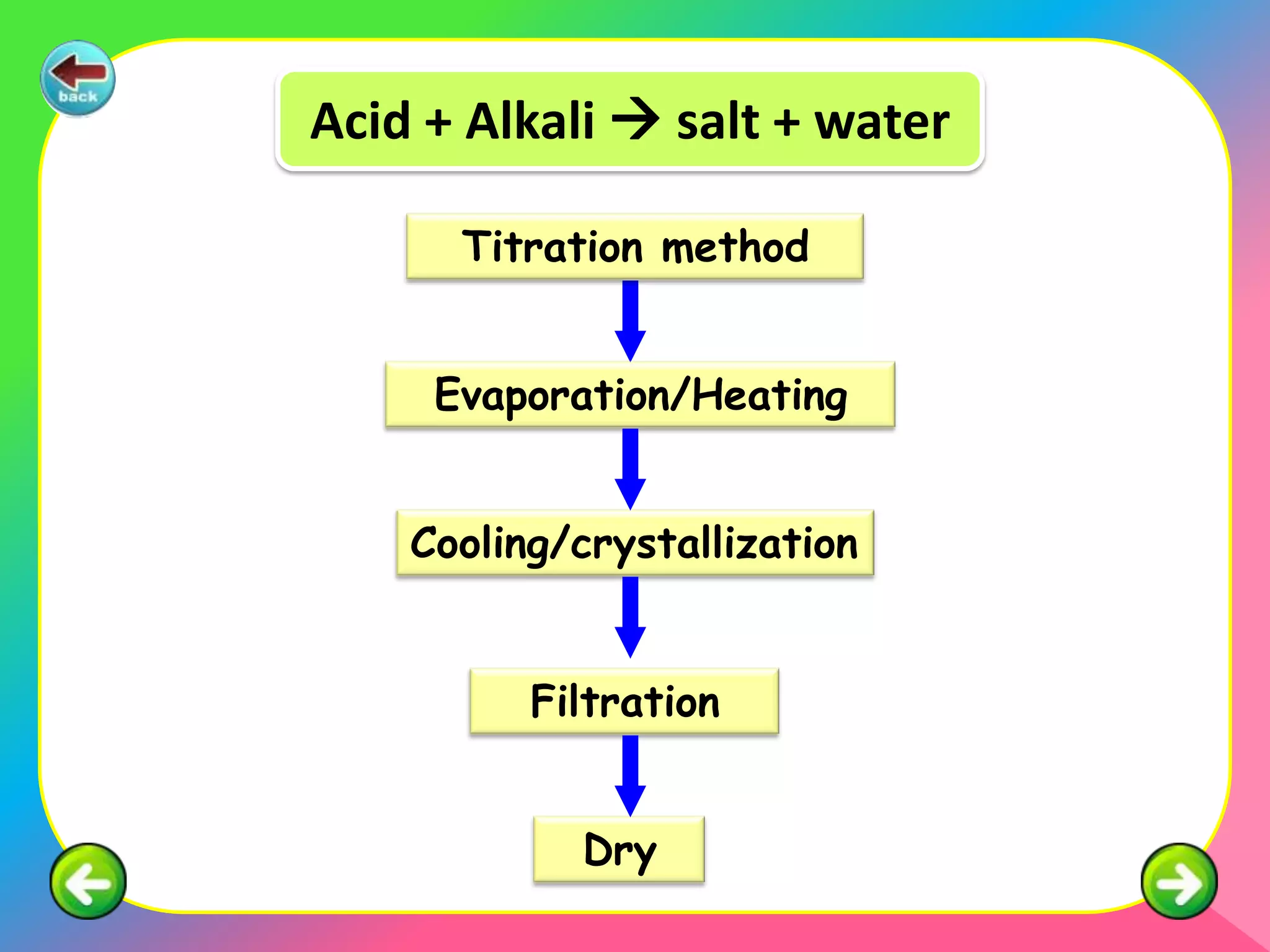
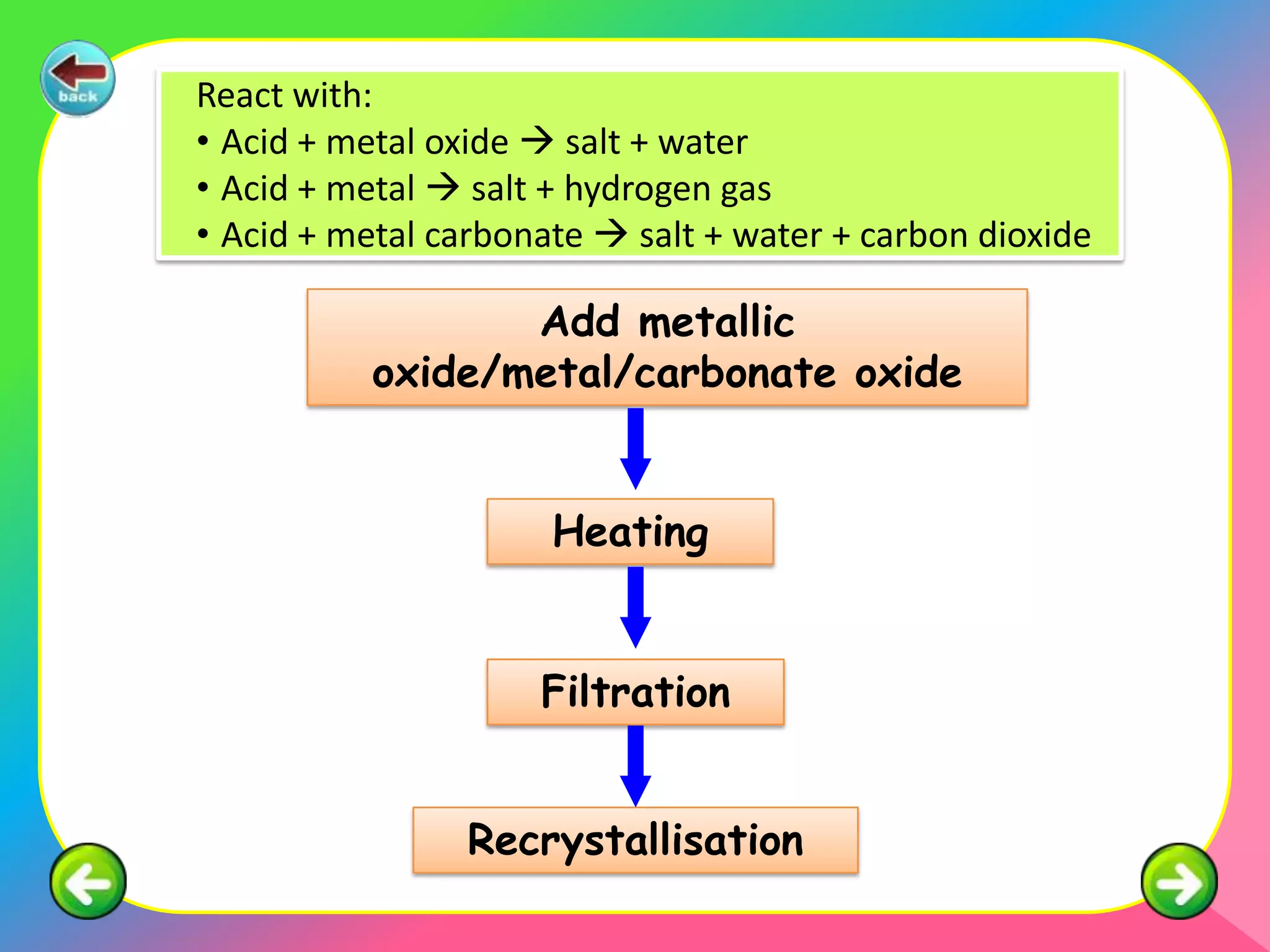
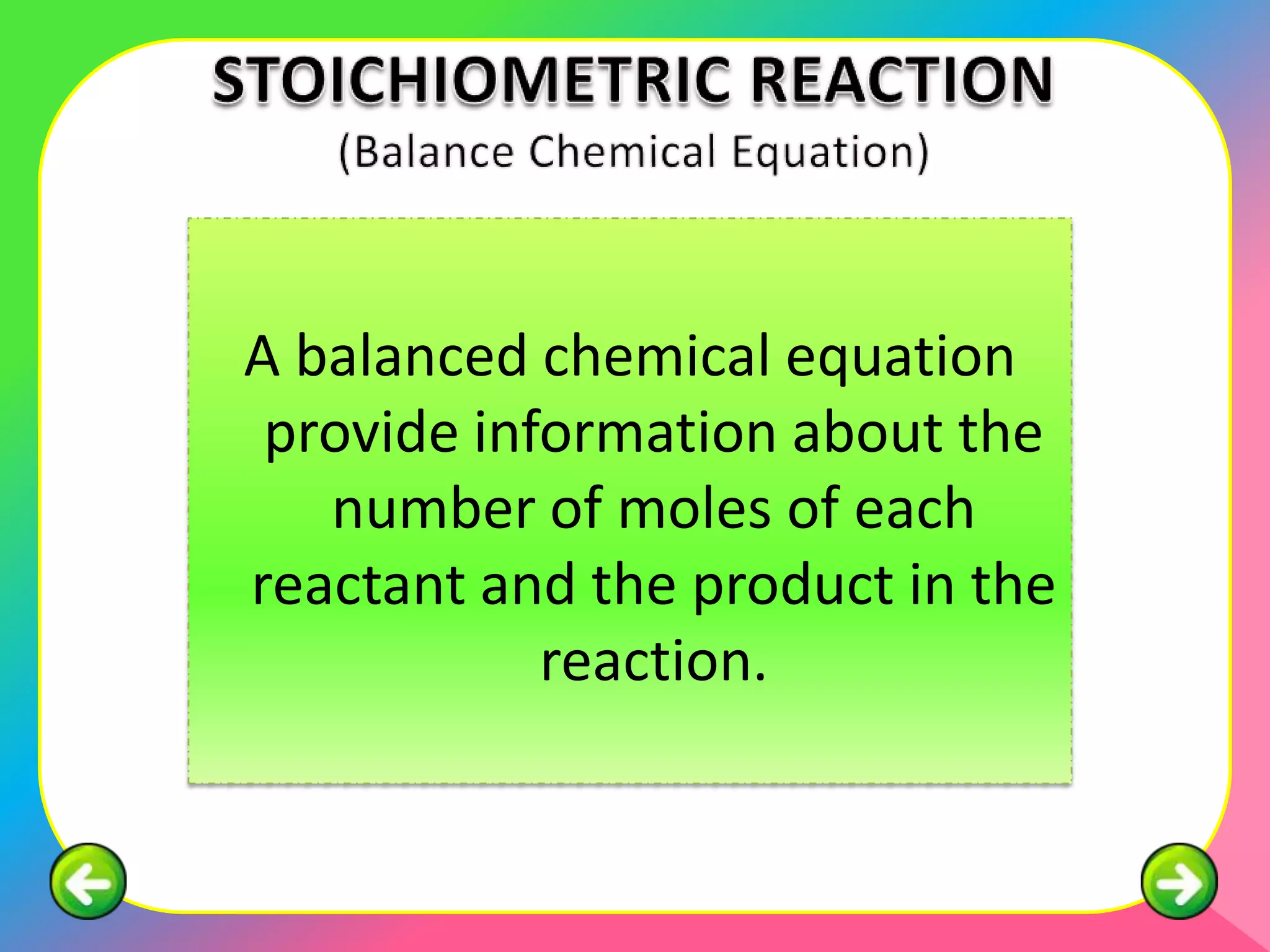
The document outlines methods for preparing specified salts through various chemical reactions. It discusses using precipitation reactions when salts are insoluble and acid-base reactions when salts contain sodium, ammonium, or potassium ions. The document provides examples of reacting acids with metal oxides, metals, and metal carbonates to produce salts. It also gives guidance on purification techniques like filtration, washing, drying, heating, cooling/crystallization after reactions. Finally, it includes 5 examples of stoichiometric calculations to determine moles, masses or volumes of reactants and products in salt forming reactions.

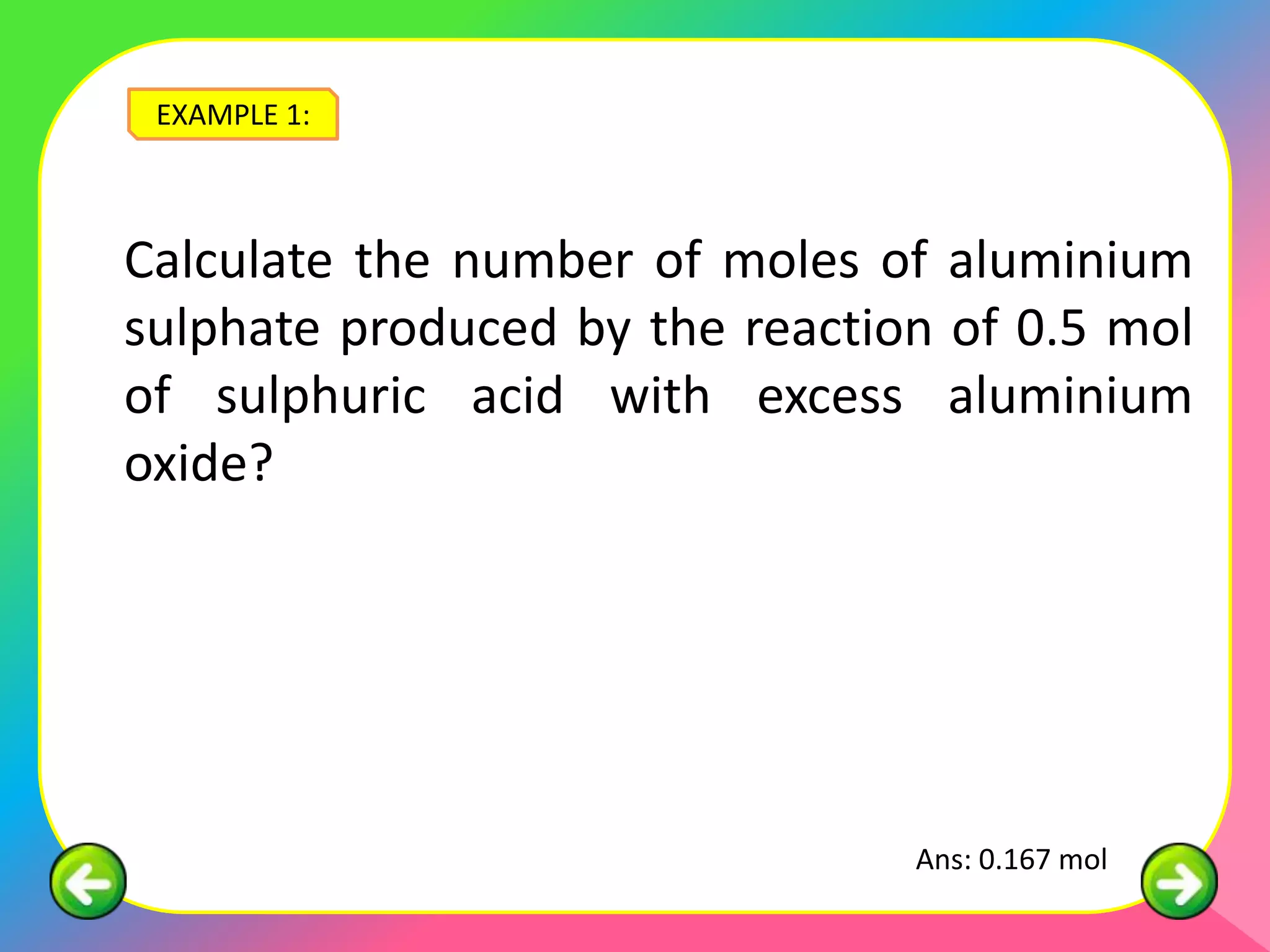
![EXAMPLE 2:
5.0 g of copper (II) carbonate powder is added
to 50cm3 of 21.9 g dm-3 hydrochloric acid.
Calculate the mass of unreacted copper (II)
carbonate.
[RAM: H, 1 ; C, 12; Cl, 35.5 ; Cu,64]
Ans: 3.14 g CuCO3](https://image.slidesharecdn.com/chapter8saltpart3-130305012035-phpapp02/75/Chapter-8-Salt-part-3-9-2048.jpg)
![EXAMPLE 3:
4.05 g of aluminium oxide powder is mixed
with excess dilute nitric acid and the mixture
is heated. Calculate the mass of aluminium
nitrate produced.
[RAM: N,14; O,16; Al, 27]
Ans: 17.04 g](https://image.slidesharecdn.com/chapter8saltpart3-130305012035-phpapp02/75/Chapter-8-Salt-part-3-10-2048.jpg)
![EXAMPLE 4:
150 cm3 of 1.0 mol dm-3 ammonia solution is
completely neutralised with phosphoric acid
using a titration methode. Calculate the mass
of ammonium phosphate formed.
[RAM: H,1 ; N,14; O,16; P,31]
Ans: 7.45 g](https://image.slidesharecdn.com/chapter8saltpart3-130305012035-phpapp02/75/Chapter-8-Salt-part-3-11-2048.jpg)
![EXAMPLE 5:
What is the volume of 2.0 mol dm-3
hydrochloric acid required to dissolved 10 g of
marble ( calcium carbonate)?
[RAM: H,1 ; O,16; C,12; Ca,40]
Ans: 100 cm3](https://image.slidesharecdn.com/chapter8saltpart3-130305012035-phpapp02/75/Chapter-8-Salt-part-3-12-2048.jpg)
