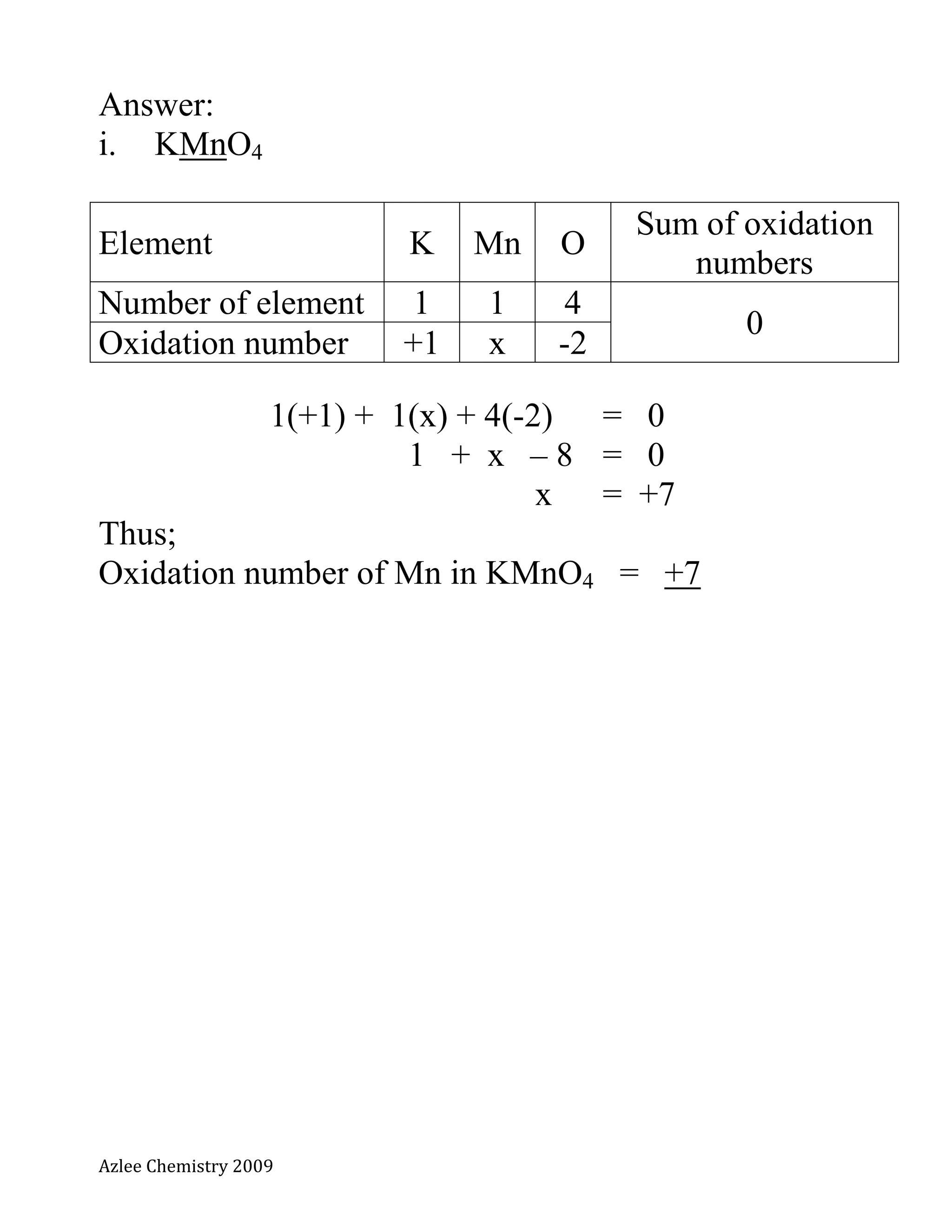
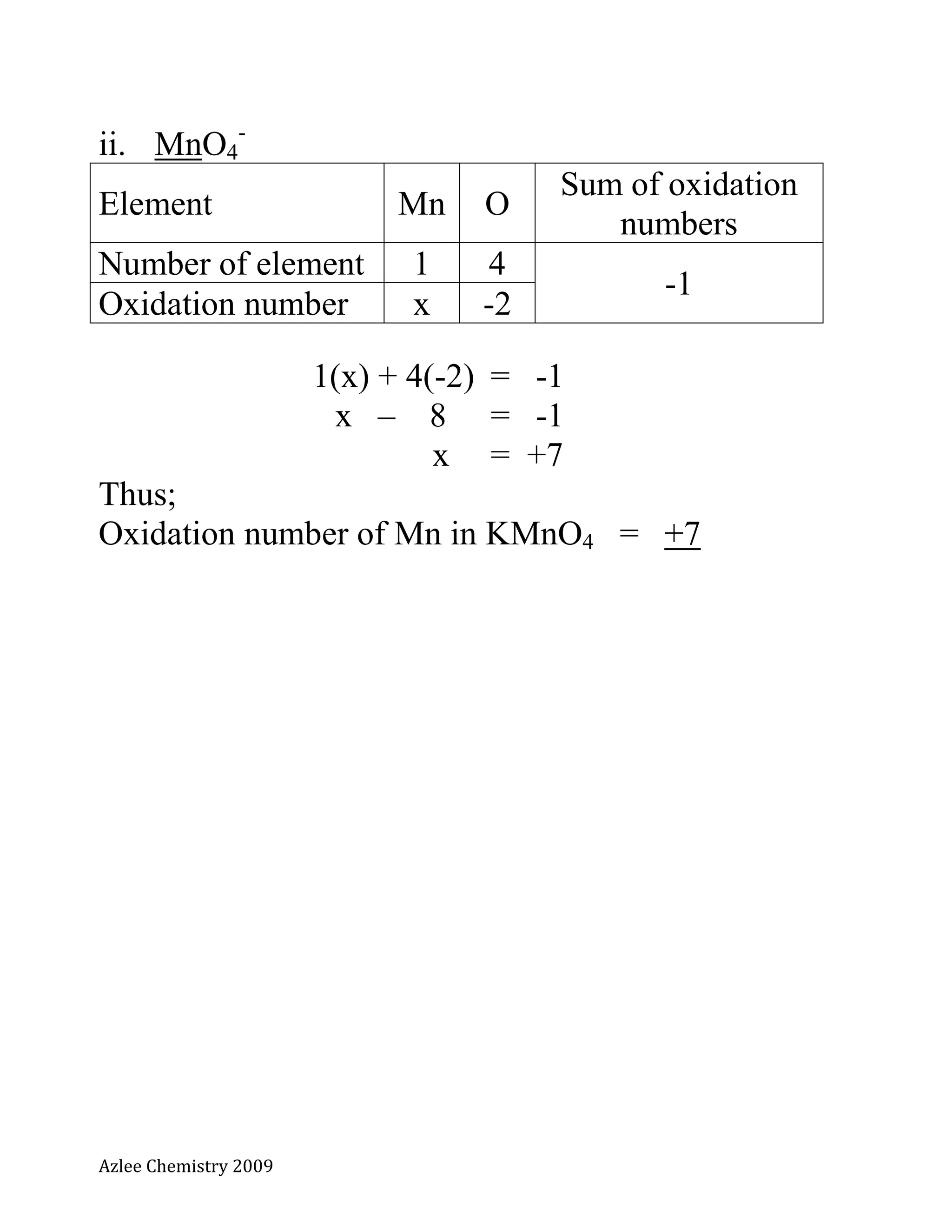
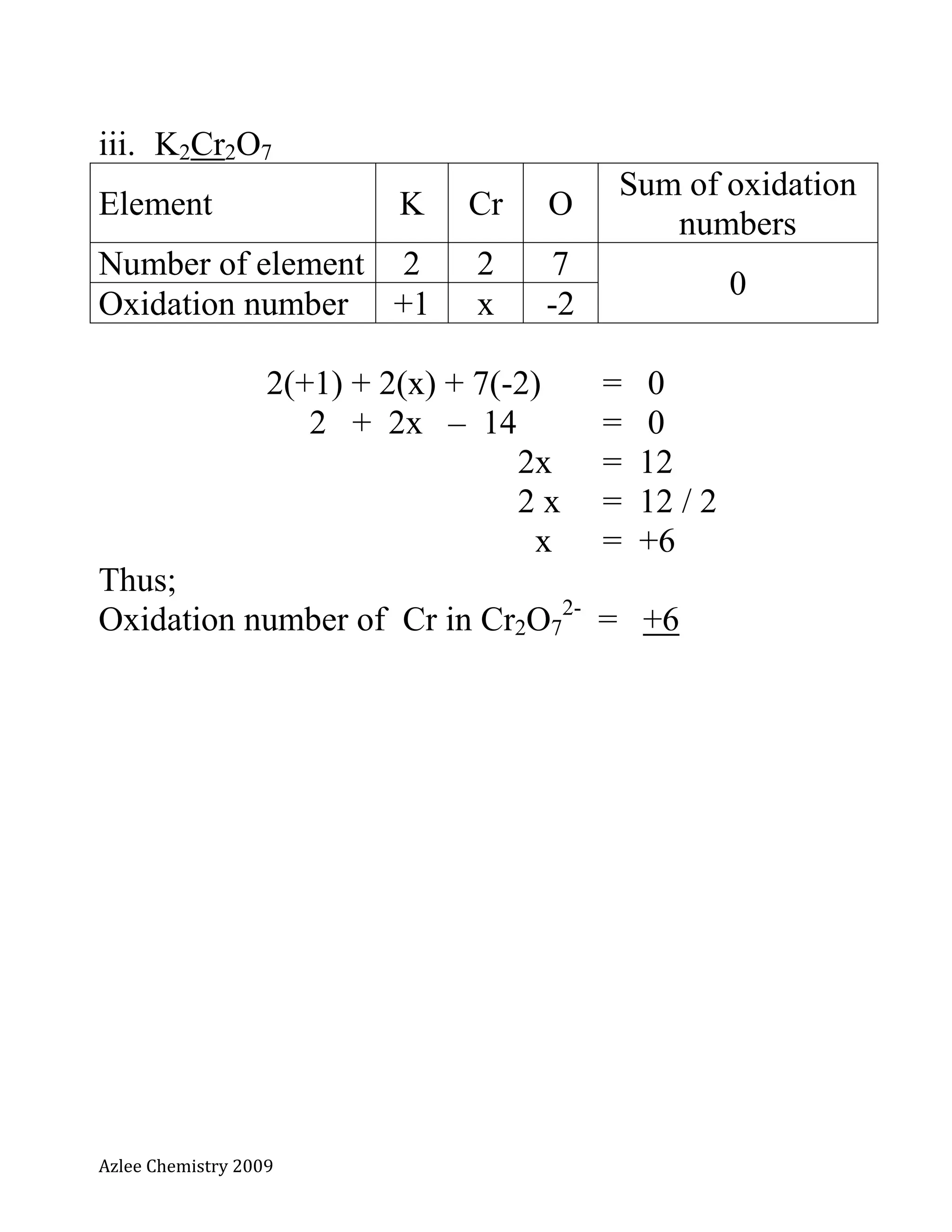
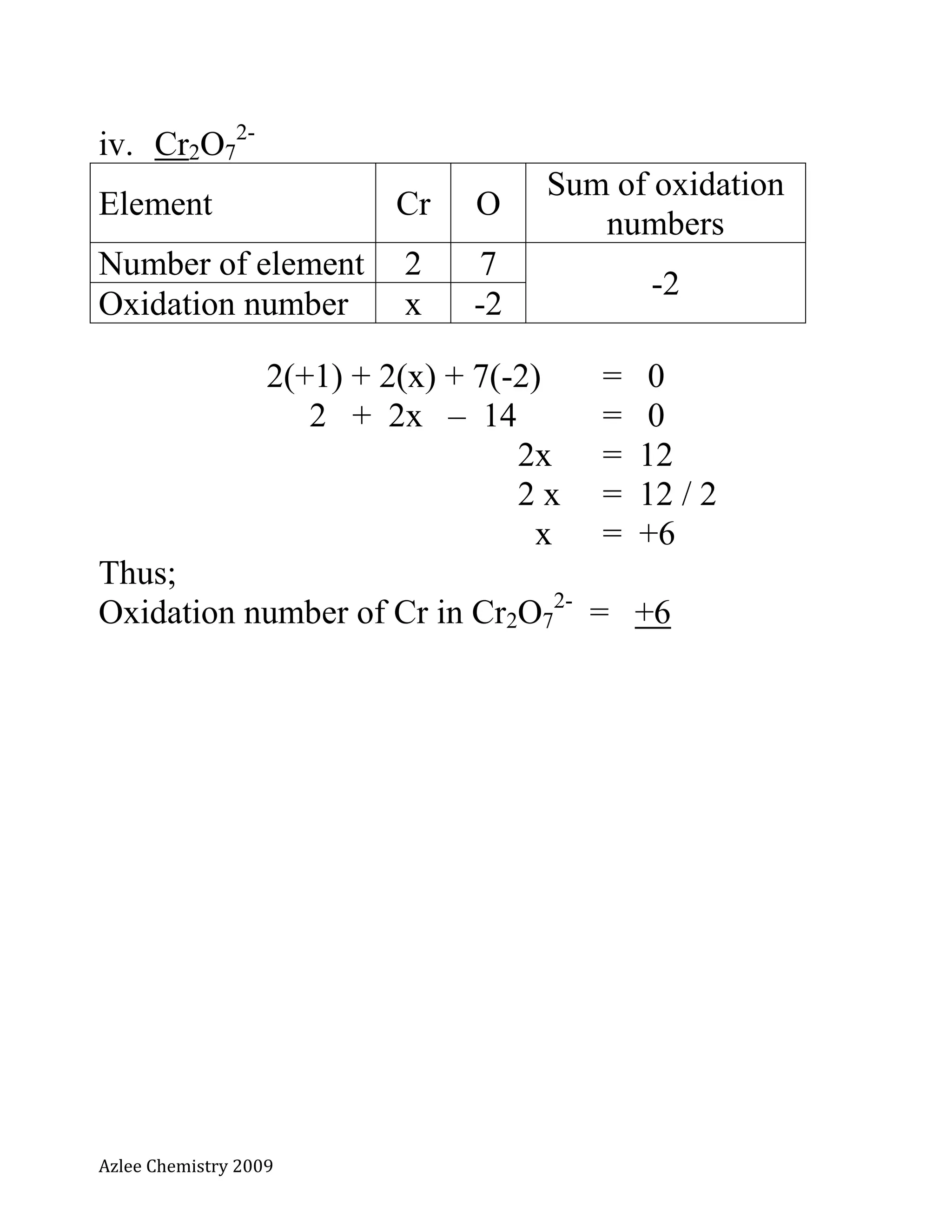
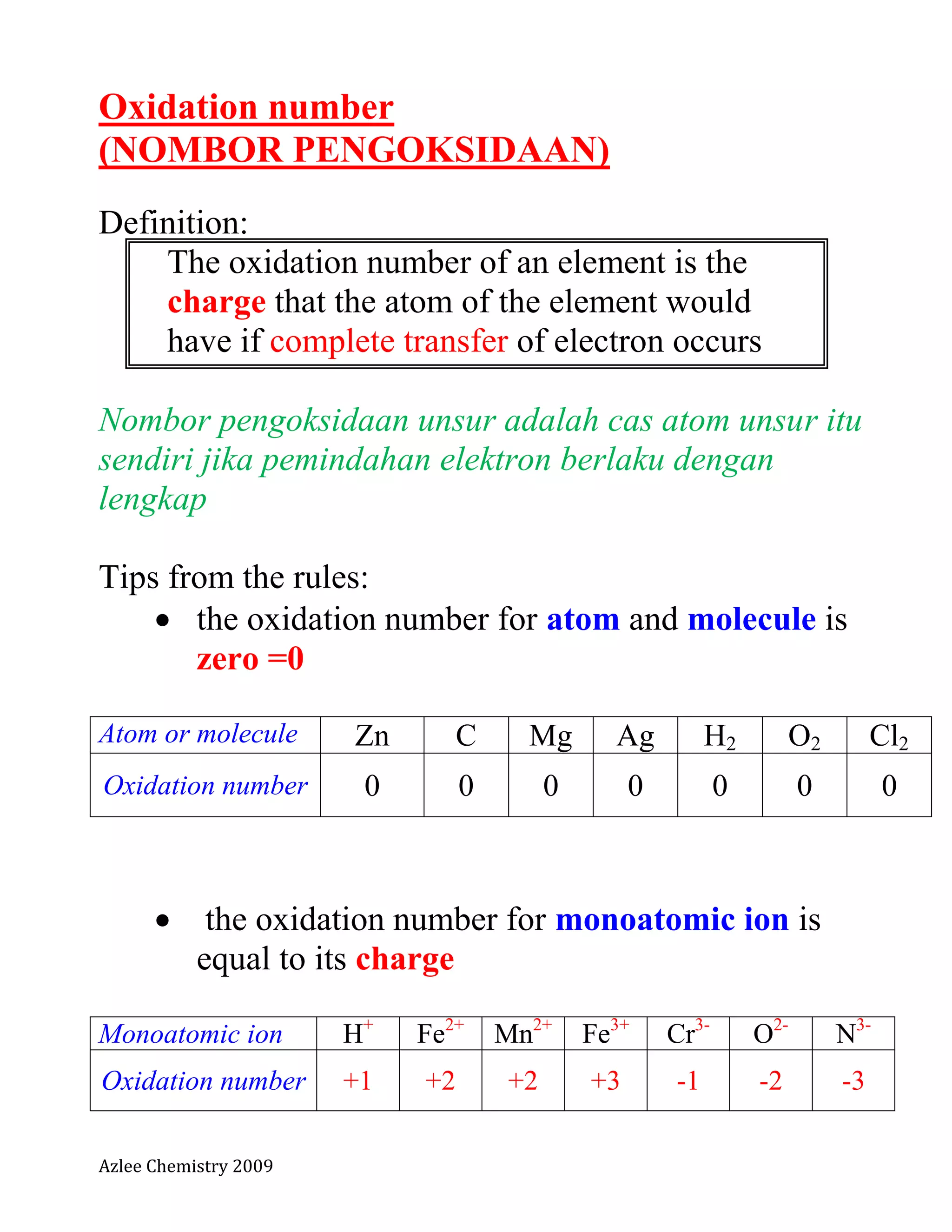
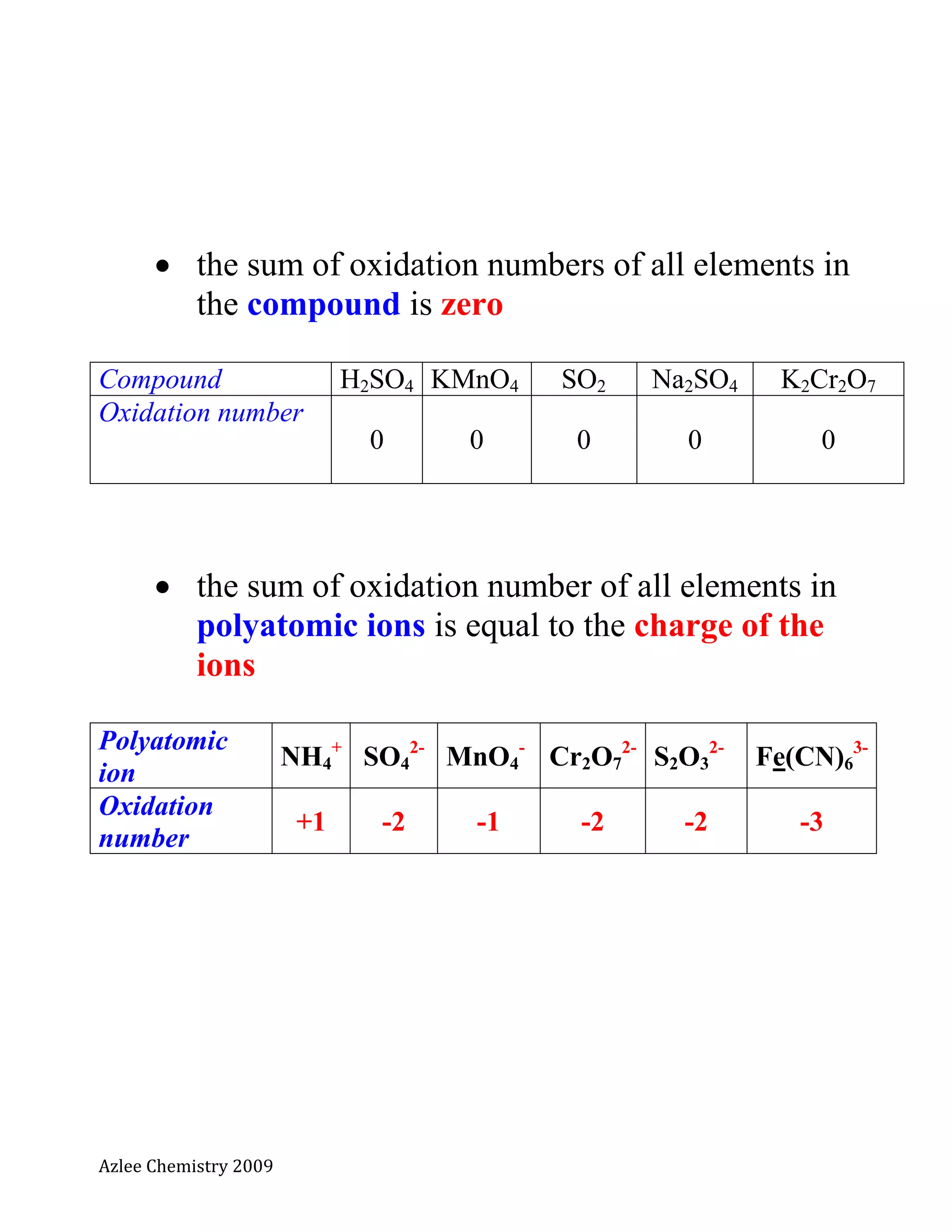
The document defines oxidation number and provides rules for determining oxidation numbers of elements in compounds and polyatomic ions. The rules state that the oxidation number of atoms is 0, ions take the charge, and the sum of oxidation numbers in compounds and polyatomic ions equals the overall charge. Examples are provided to demonstrate applying the rules to calculate the oxidation number of underlined elements in various compounds and polyatomic ions.
![Azlee Chemistry 2009
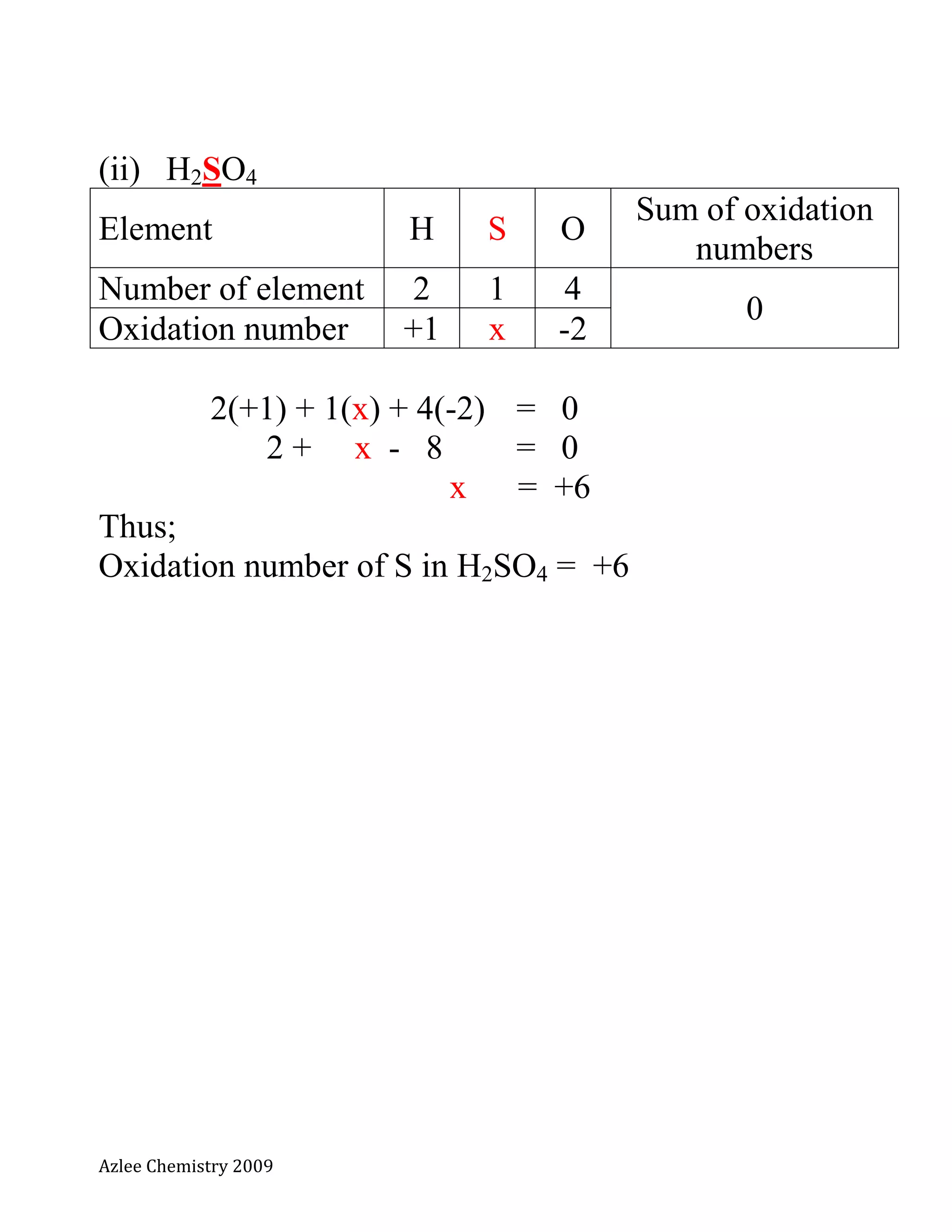
Calculate the oxidation numbers for the underlined
elements.
(i) SO2 [compound]
[refer to rules above]
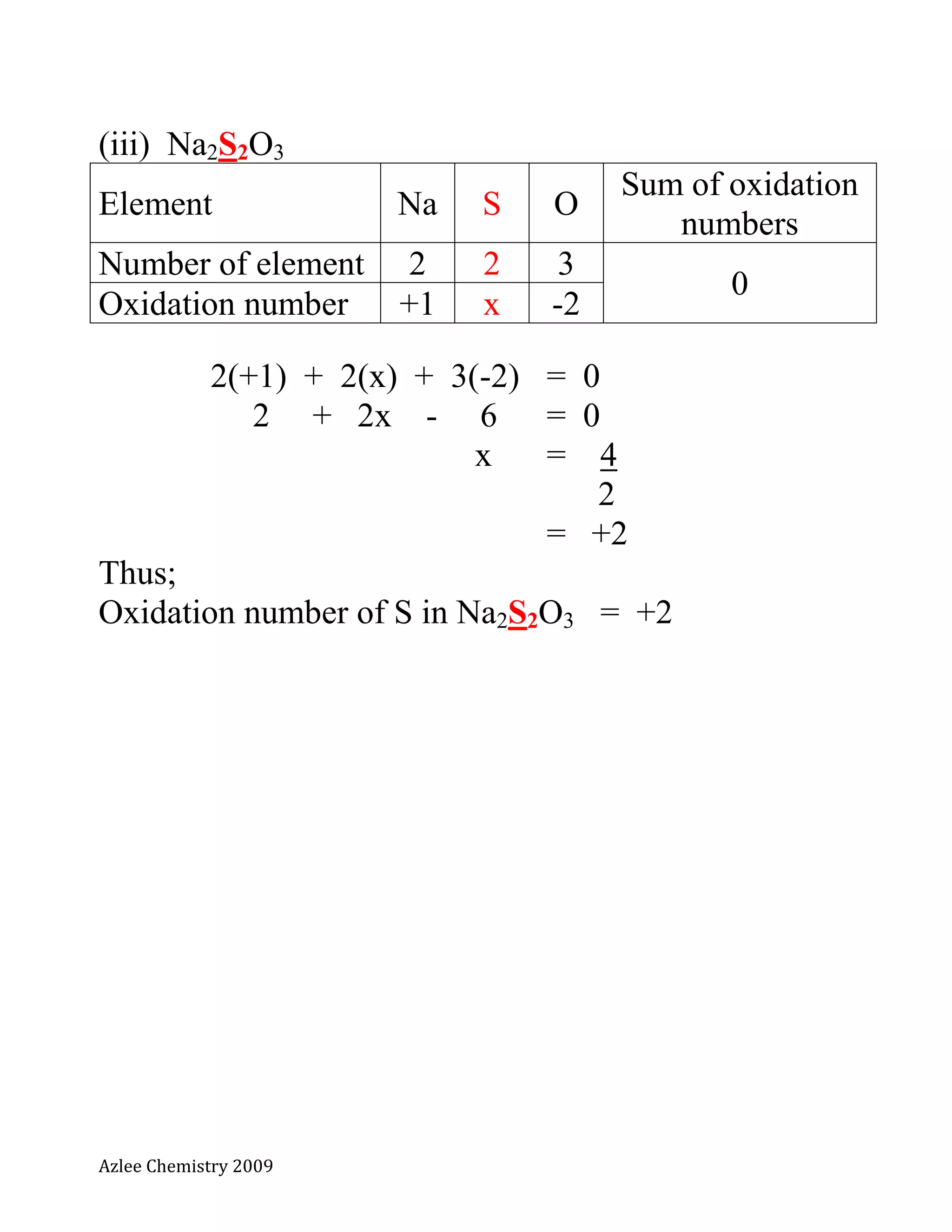
Element S O
Sum of oxidation
numbers
Number of element 1 2
0
Oxidation number x -2
[let the unknown oxidation number is equal to x]
1(x) + 2(-2) = 0
x - 4 = 0
x = +4
Thus;
Oxidation number of S in SO2 = +4
Latihan
Dapatkan nombor pengoksidaan bagi
H2SO4
Na2S2O3](https://image.slidesharecdn.com/notakimiat5nopengoksidaan-130718204742-phpapp02/75/Nota-kimia-t5-no-pengoksidaan-3-2048.jpg)
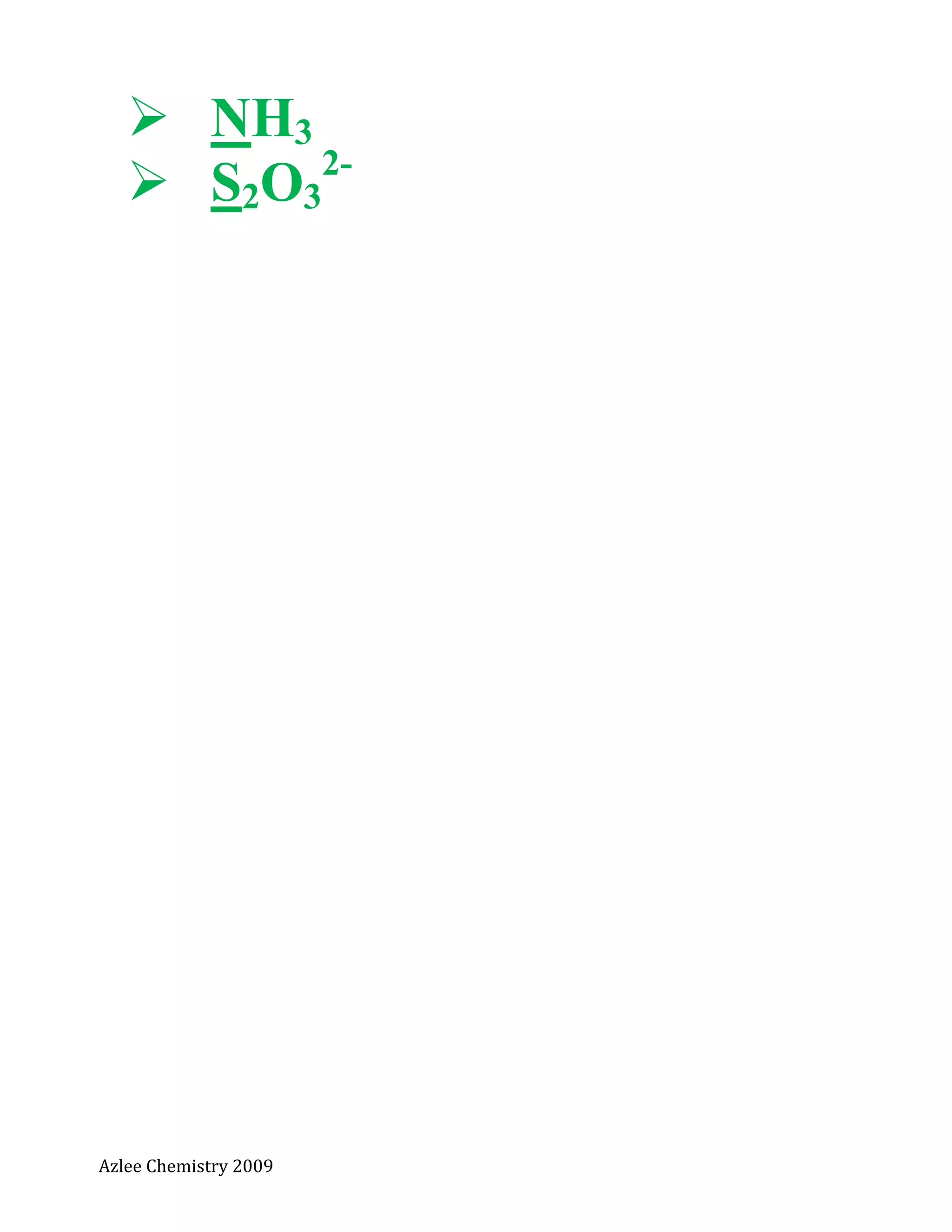
![Azlee Chemistry 2009
(v) S2O3
2-
[polyatomic ion]
[refer to rules above]
Element S O
Sum of oxidation
numbers
Number of element 2 3
-2
Oxidation number x -2
2(x) + 3(-2) = -2
2x - 6 = -2
x = (6 – 2) /2
x = +2
Thus;
Oxidation number of S in S2O3
2-
= +2
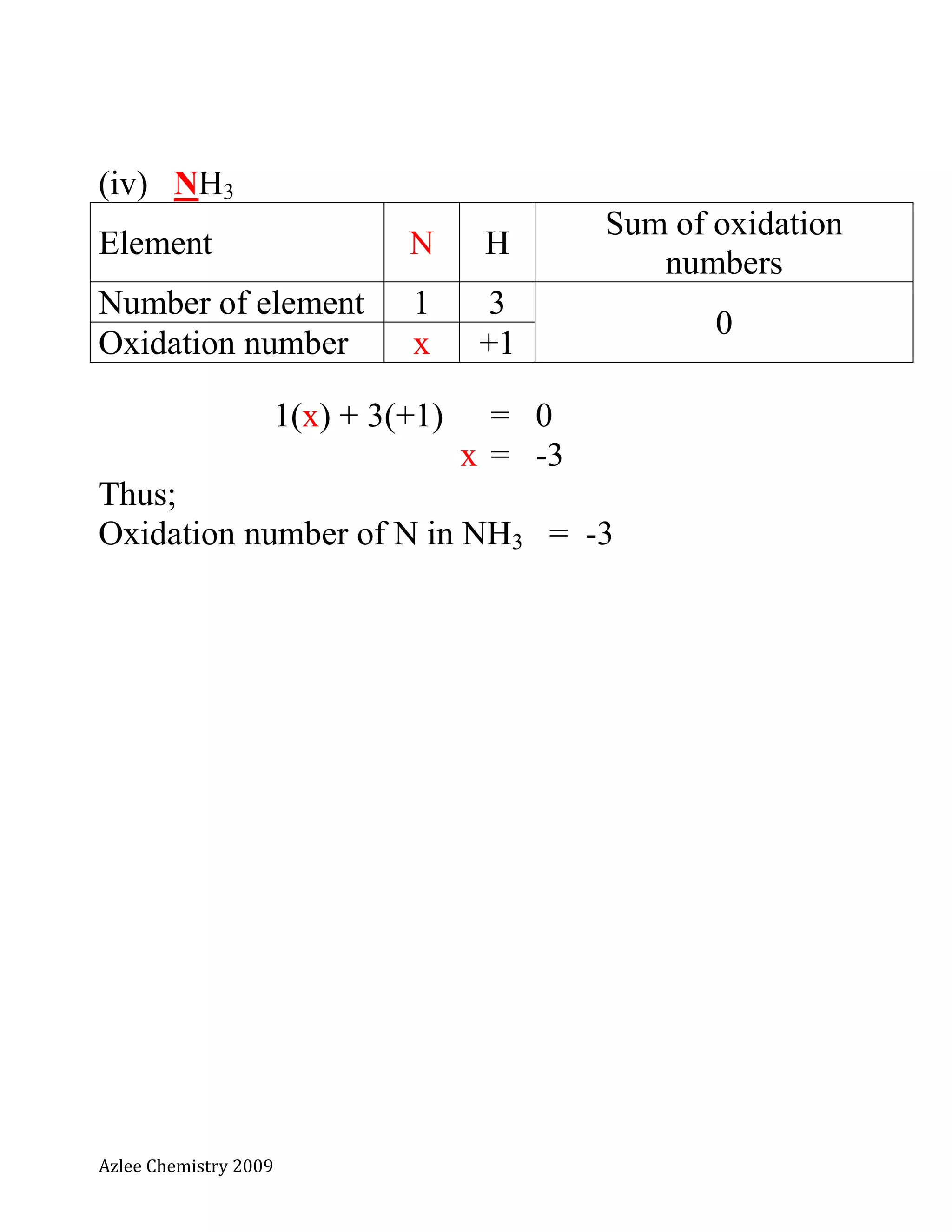
(vi) NH4
+
: ammonium ion [polyatomic ion]
Element N H
Sum of oxidation
numbers
Number of element 1 4
+1
Oxidation number x +1
x(1) + 4(+1) = +1
x + 4 = +1
x = 1 - 4
x = -3
Thus;
Oxidation number of N in NH4
+
= -3](https://image.slidesharecdn.com/notakimiat5nopengoksidaan-130718204742-phpapp02/75/Nota-kimia-t5-no-pengoksidaan-8-2048.jpg)