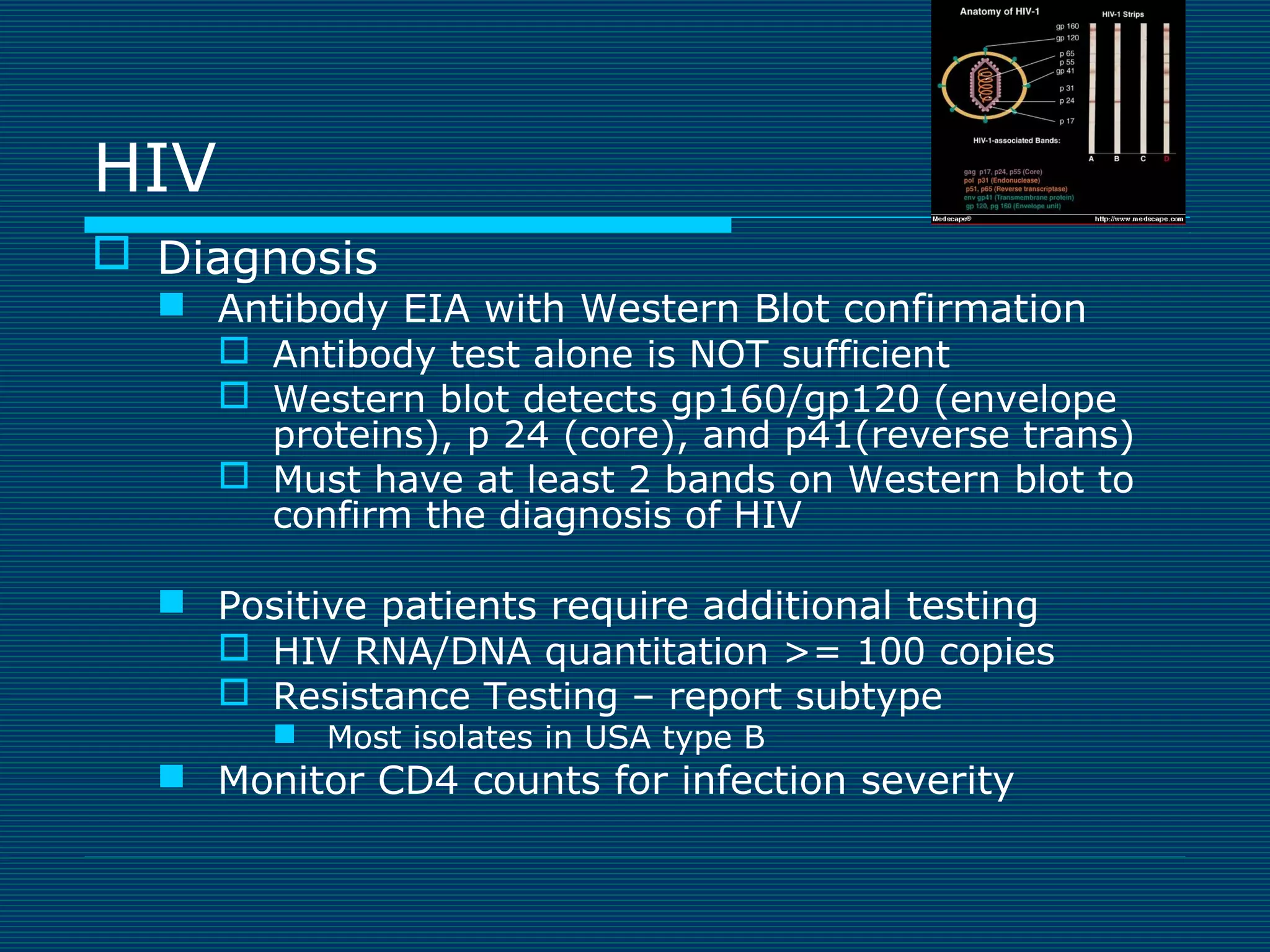
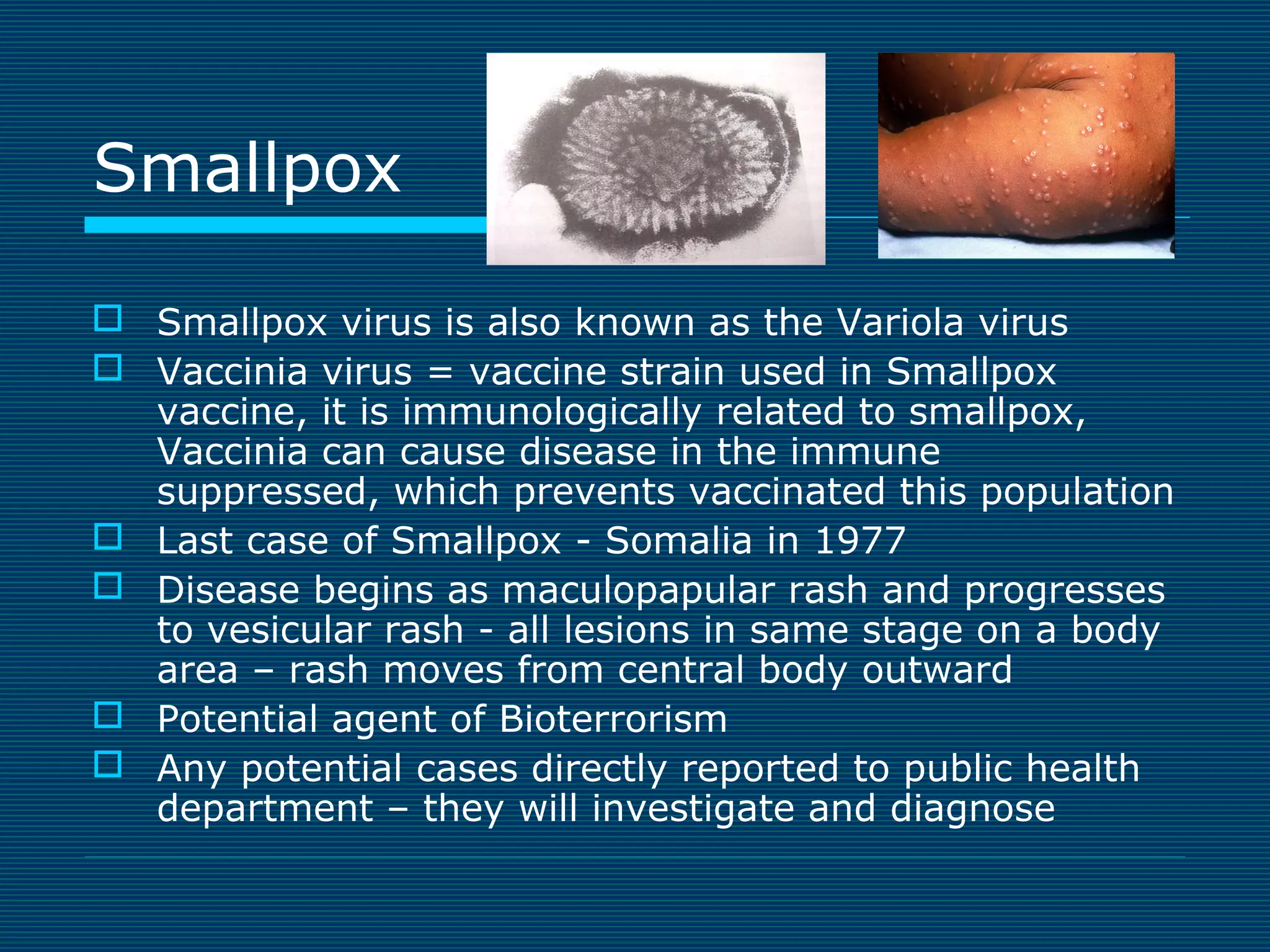
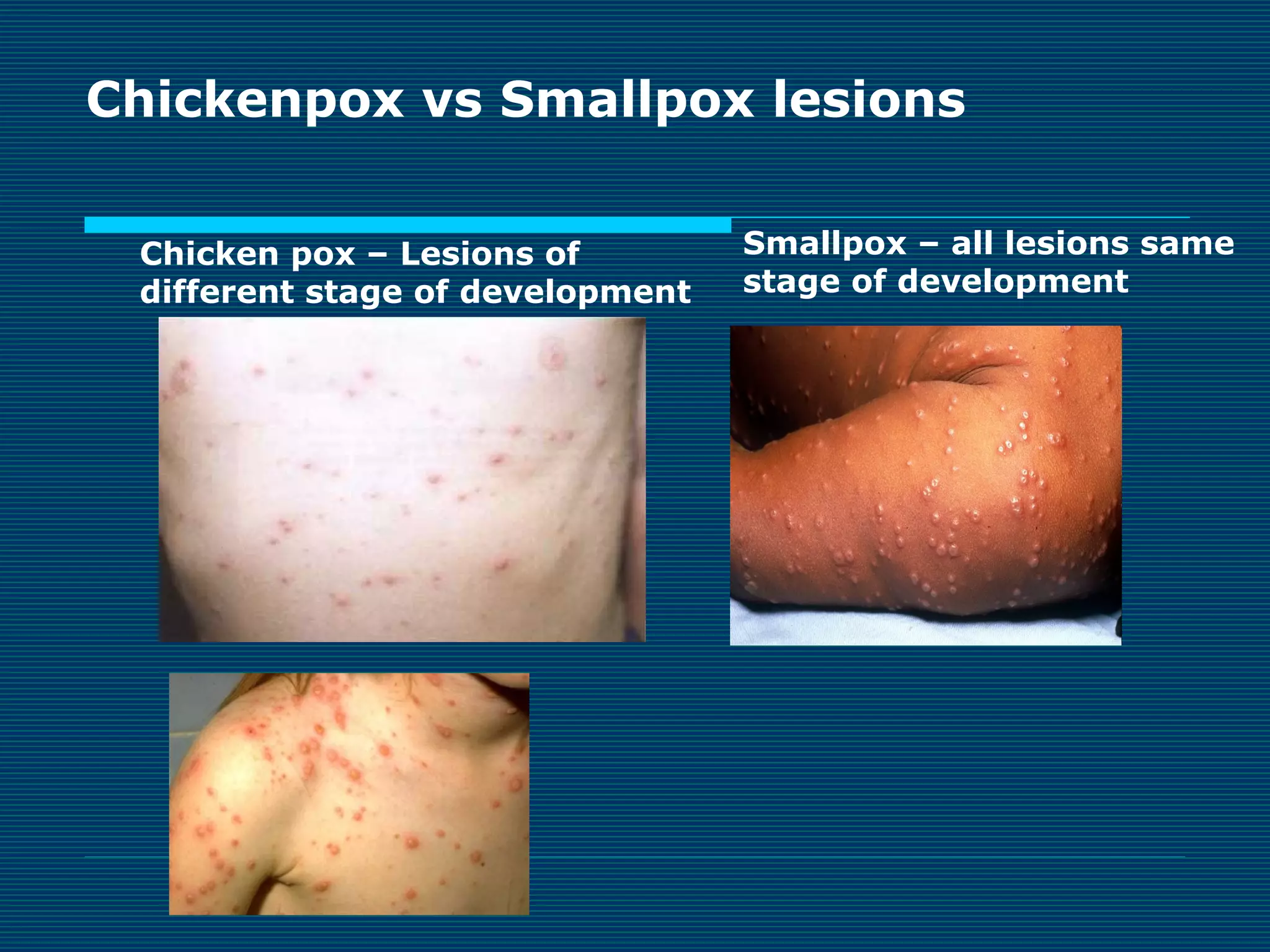
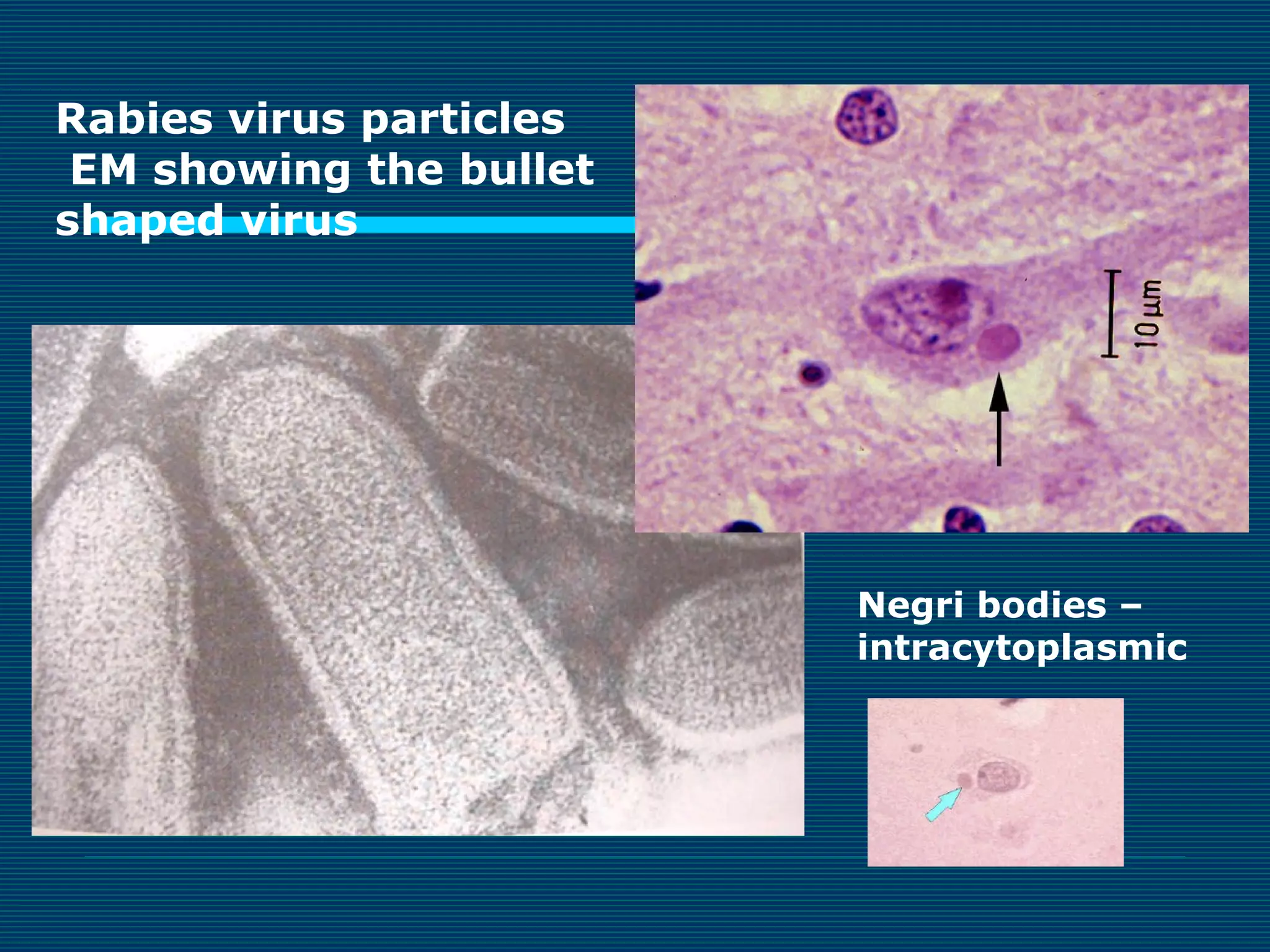
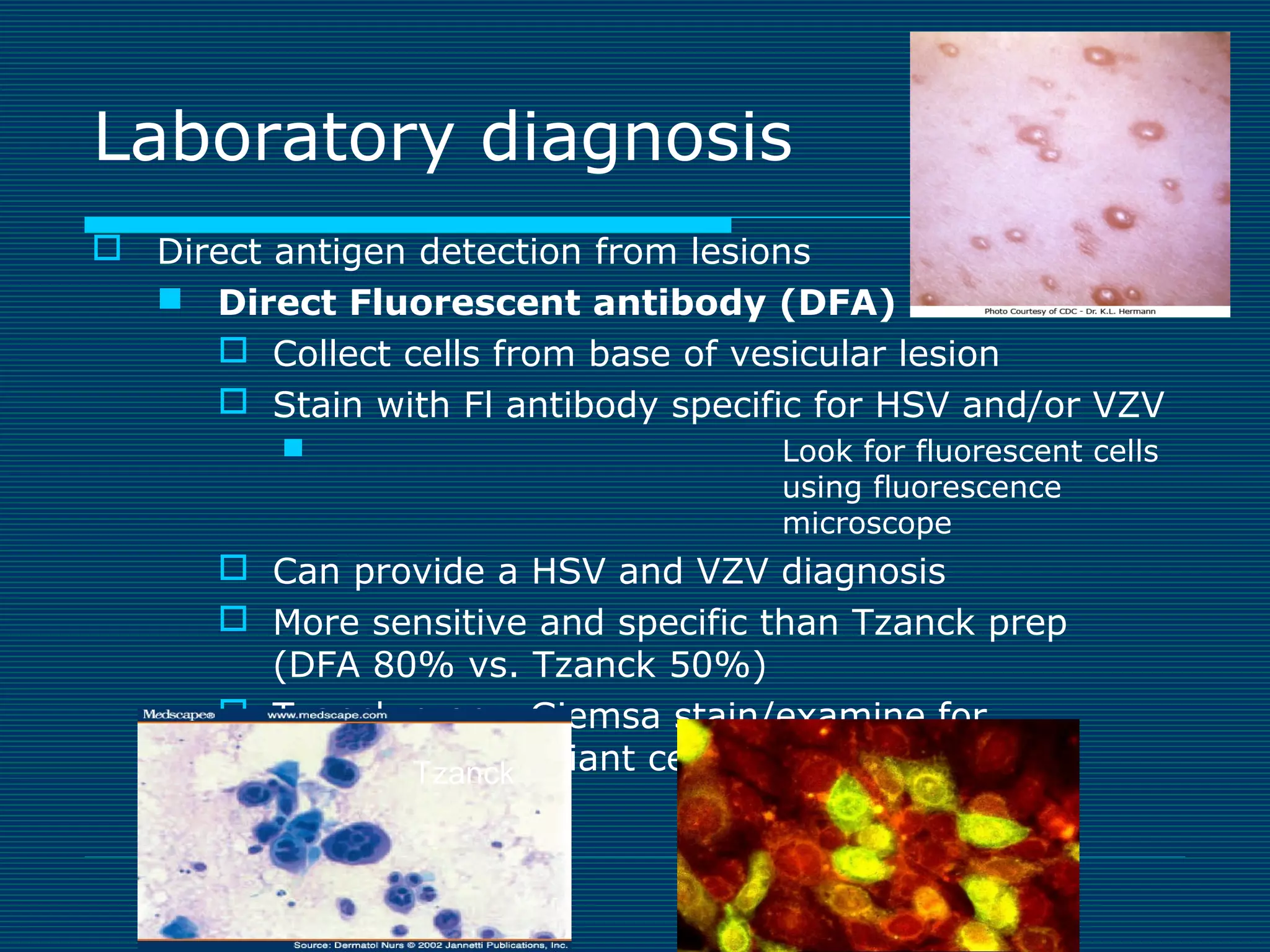
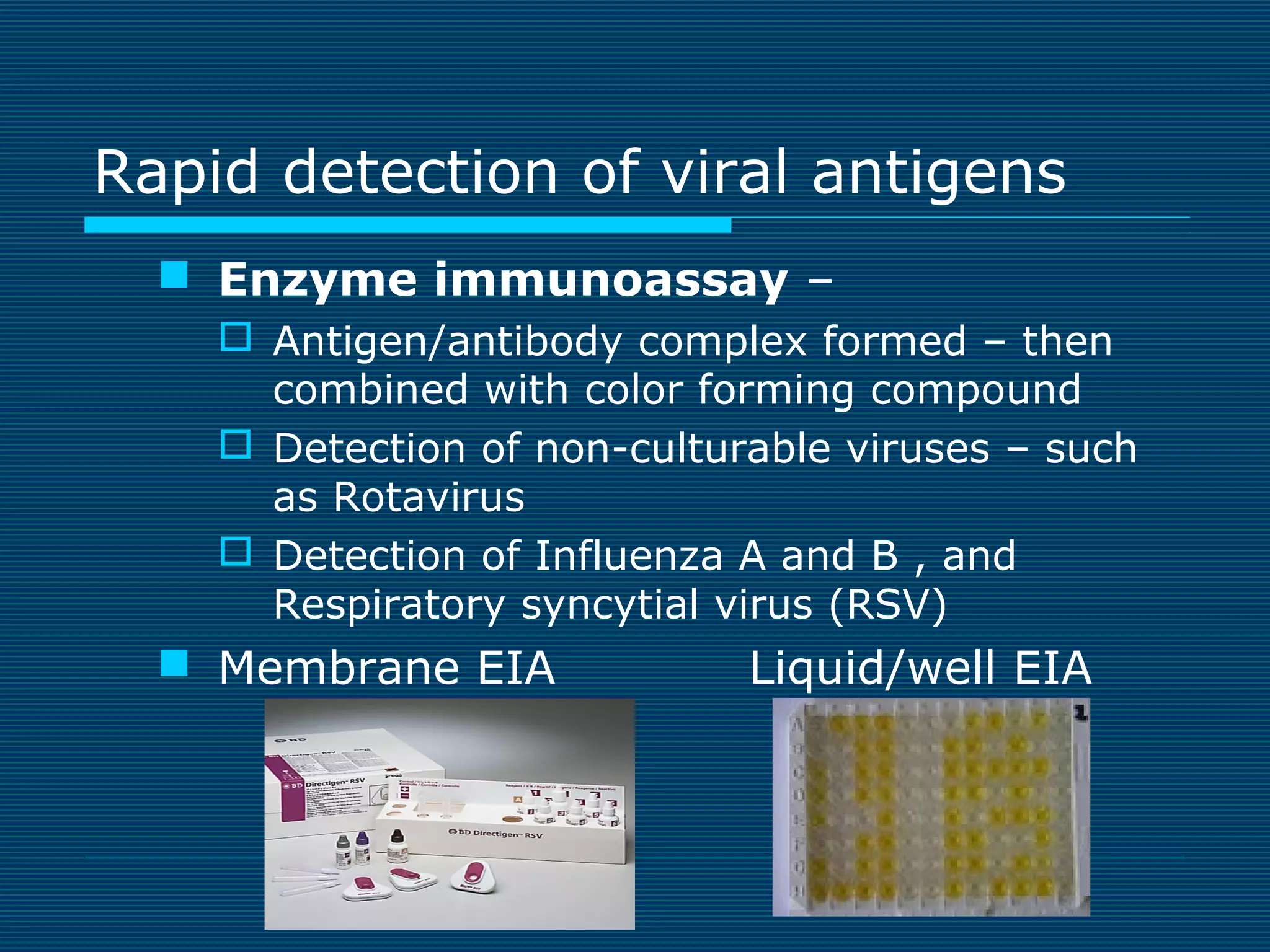
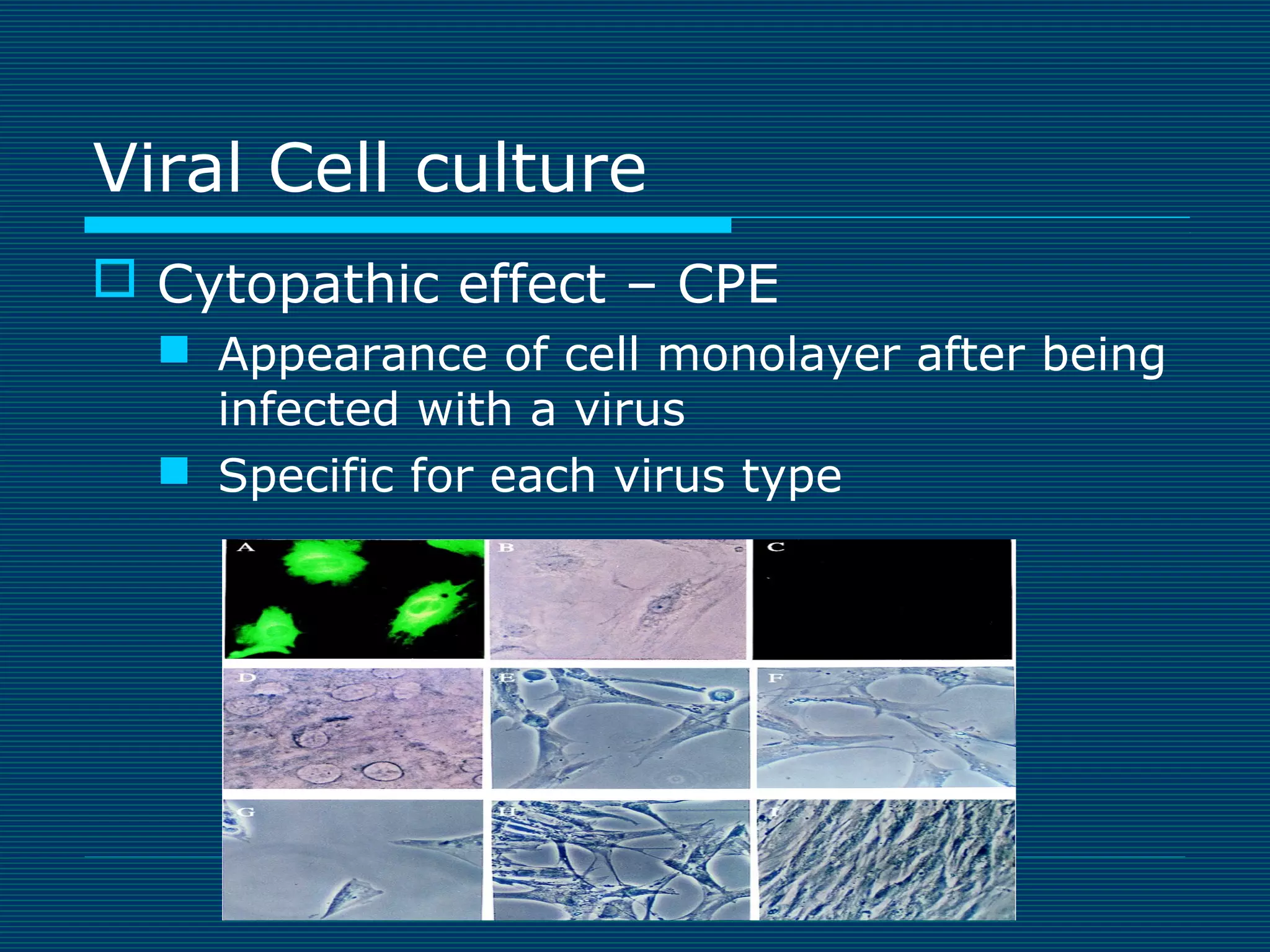
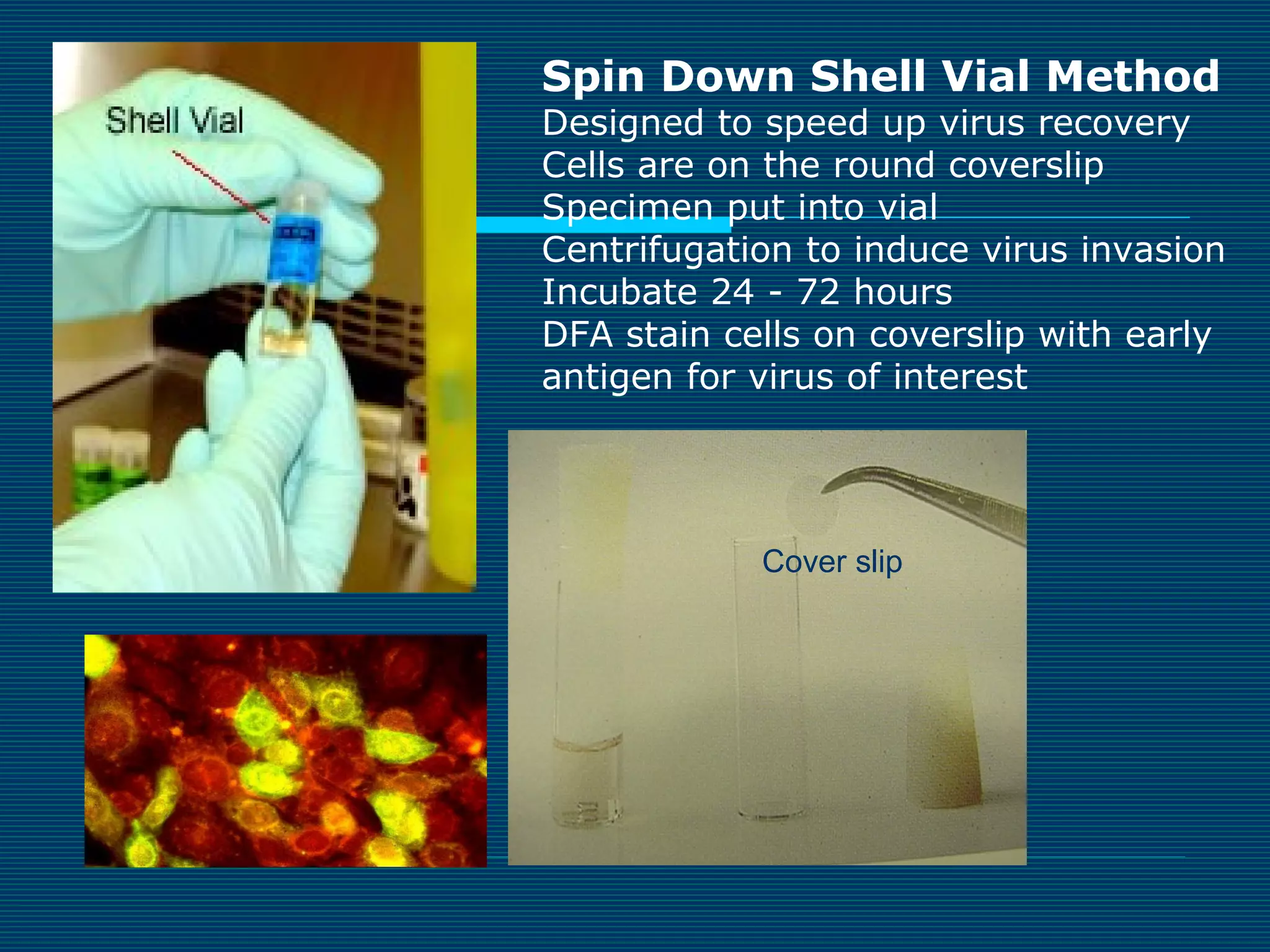
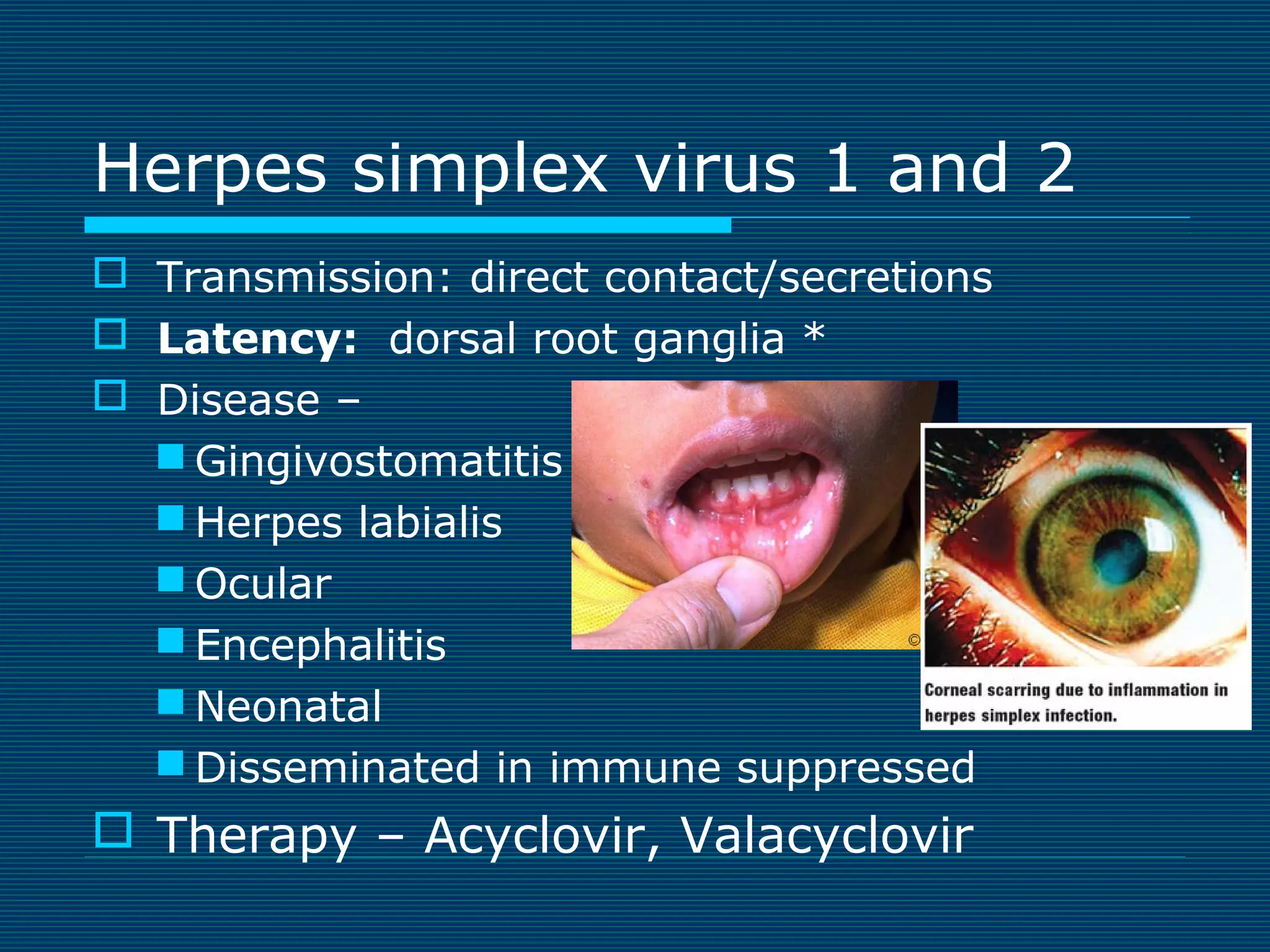
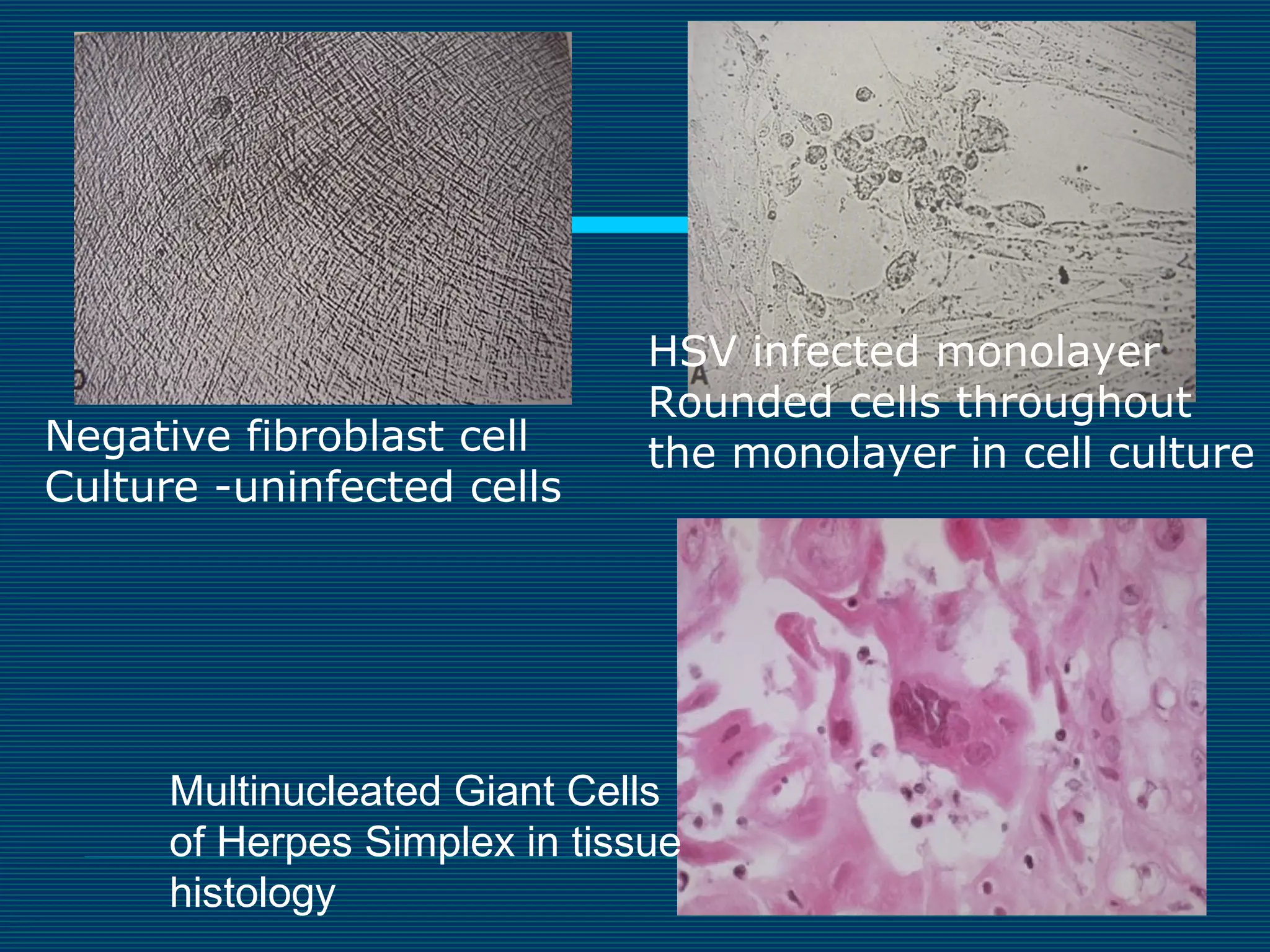
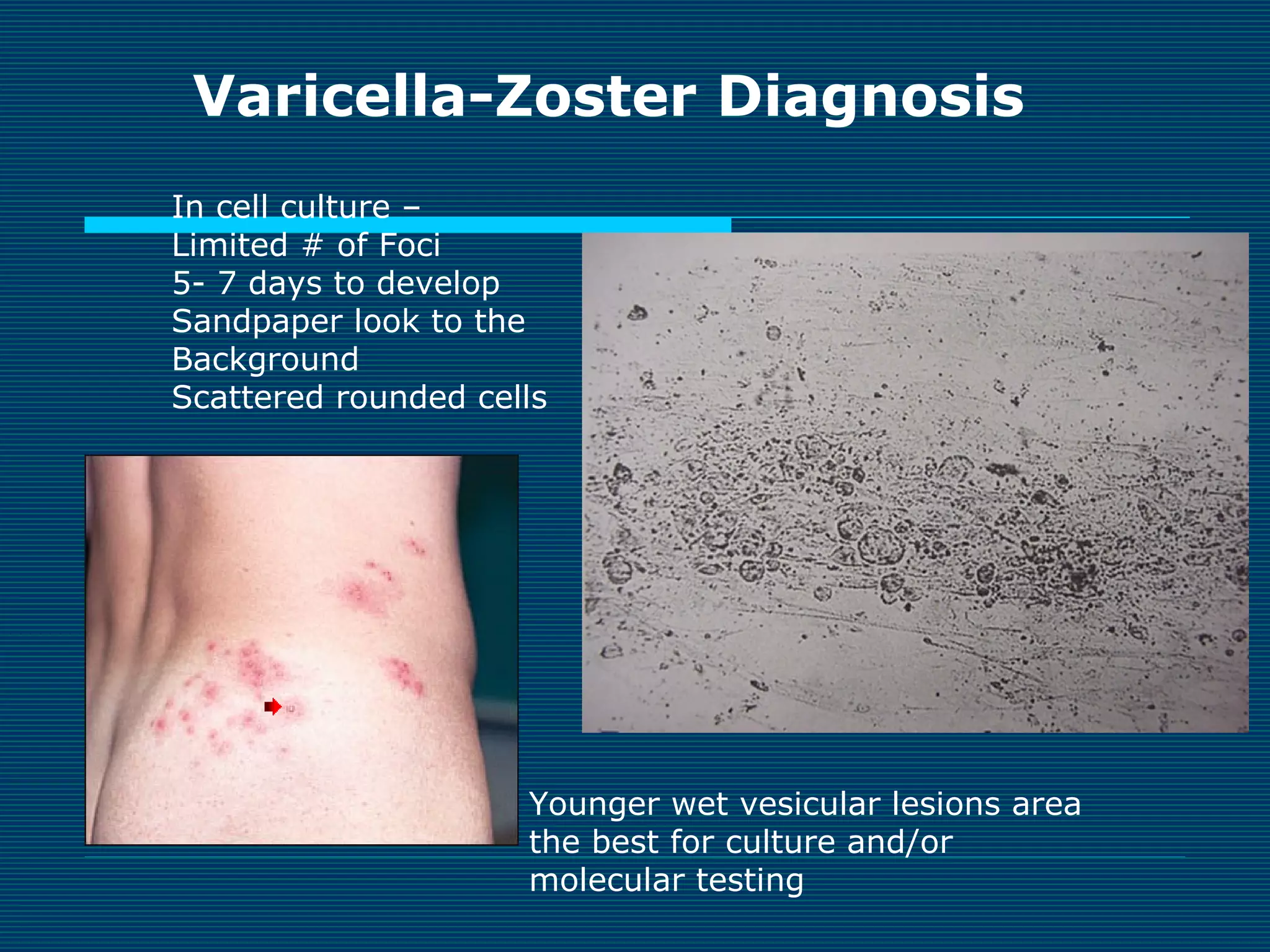
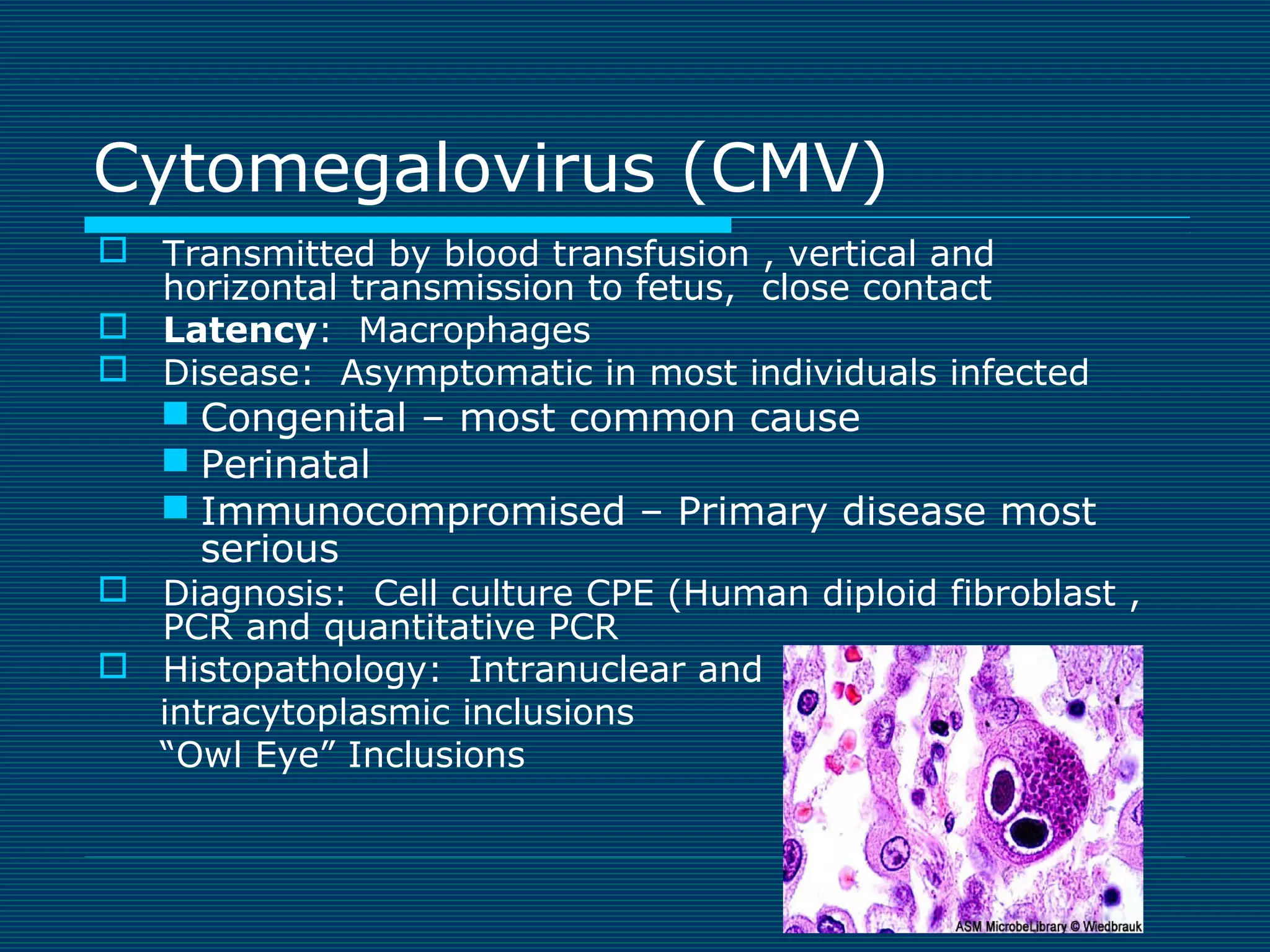
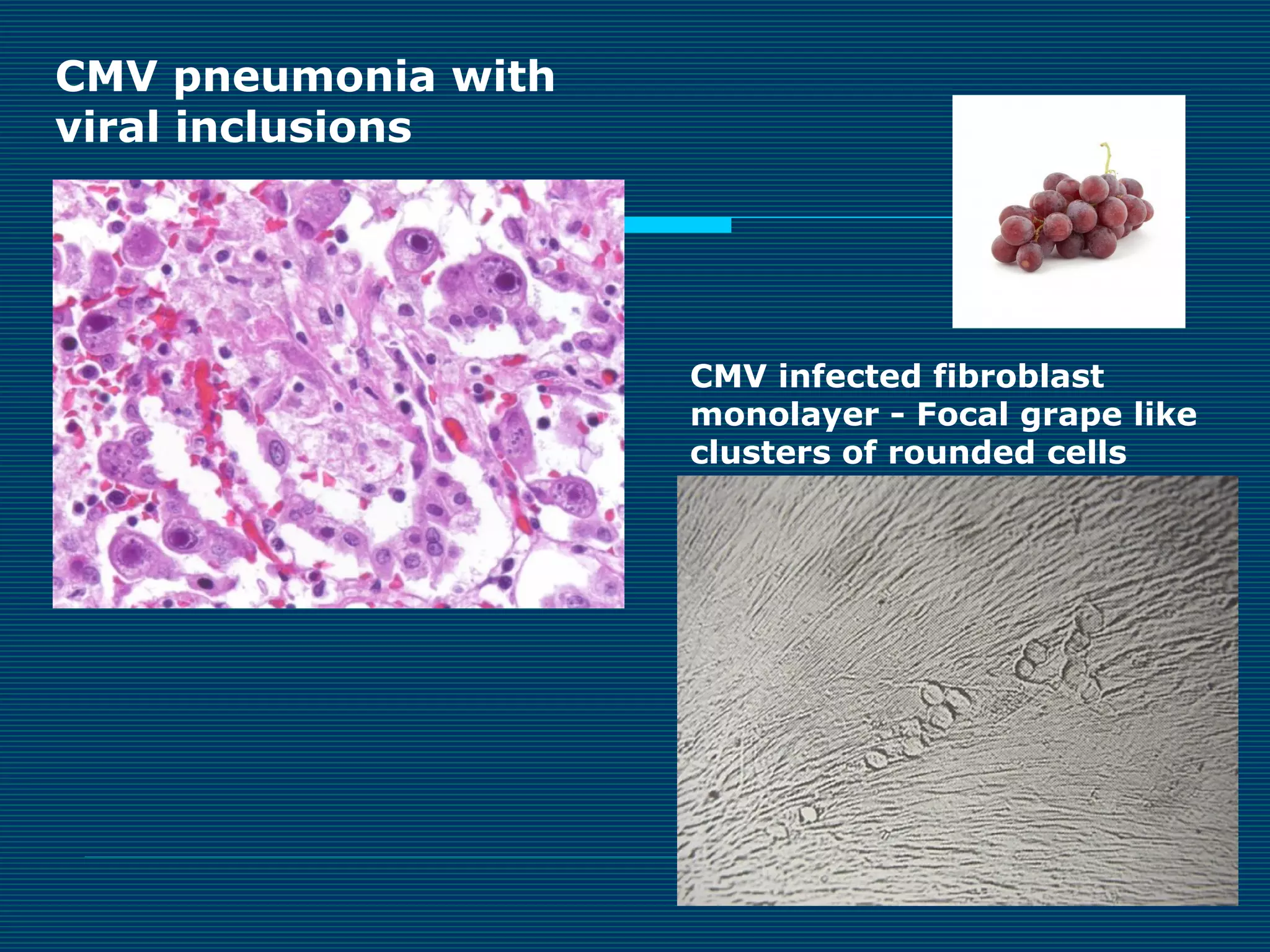
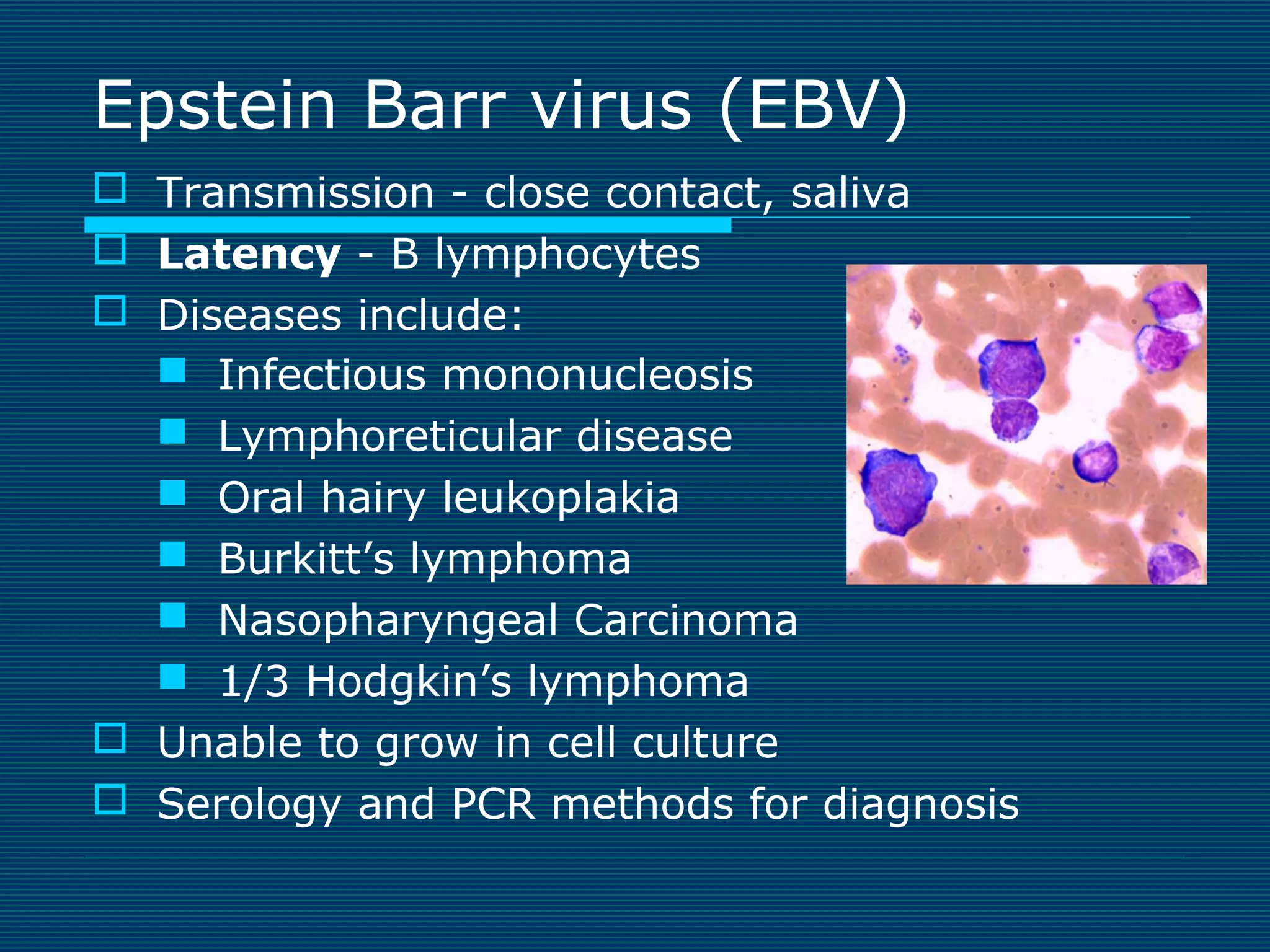
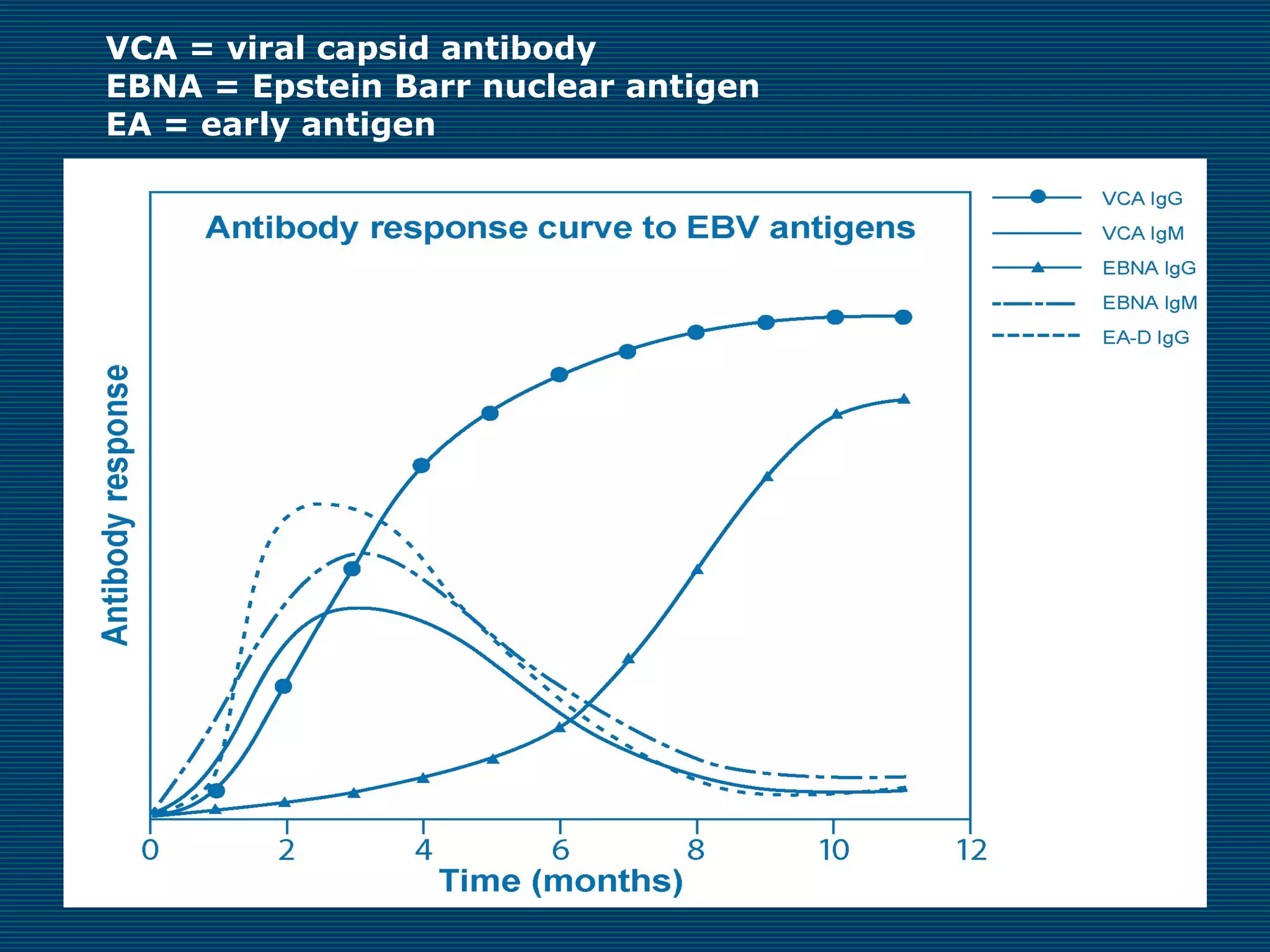
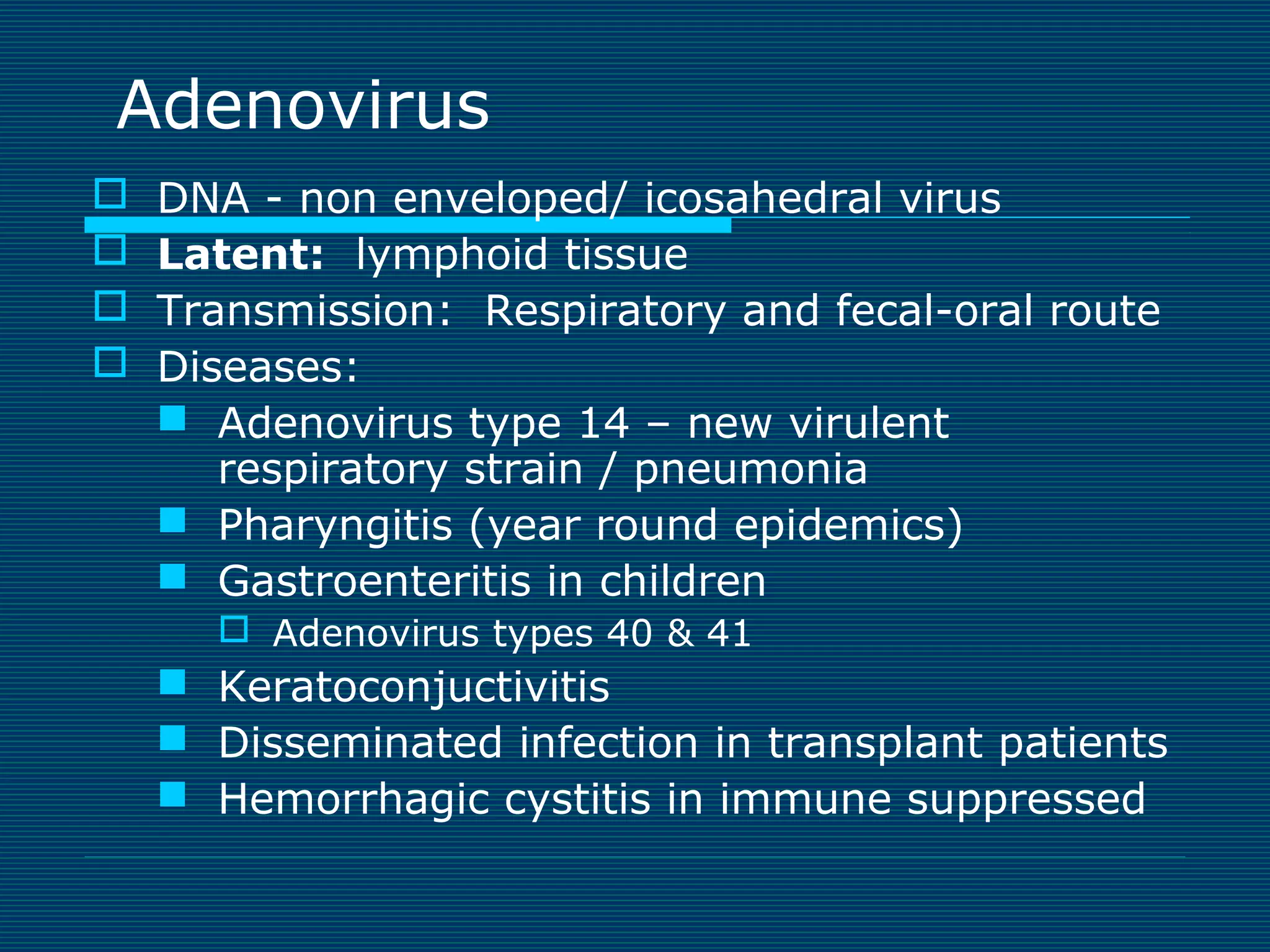
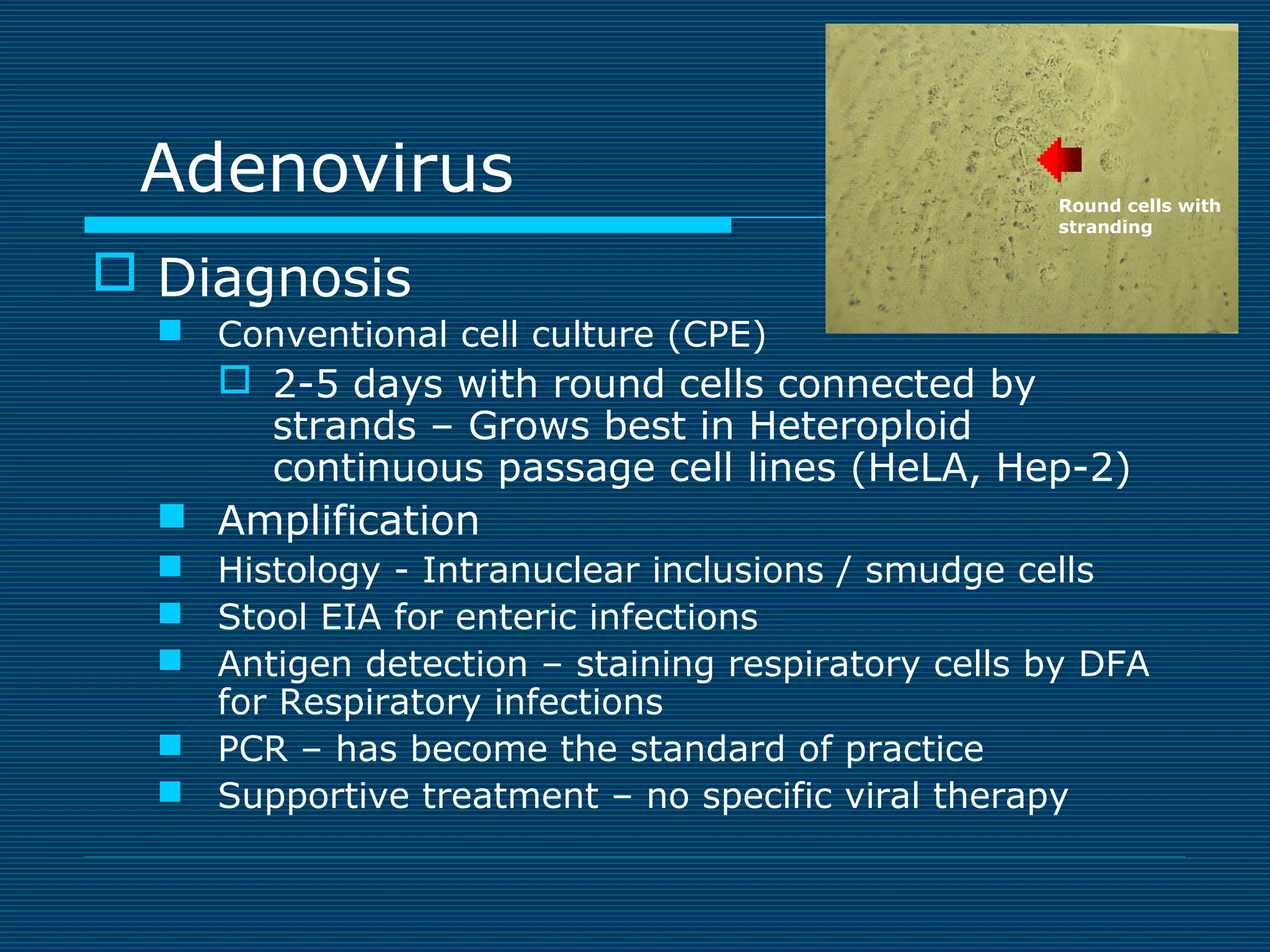
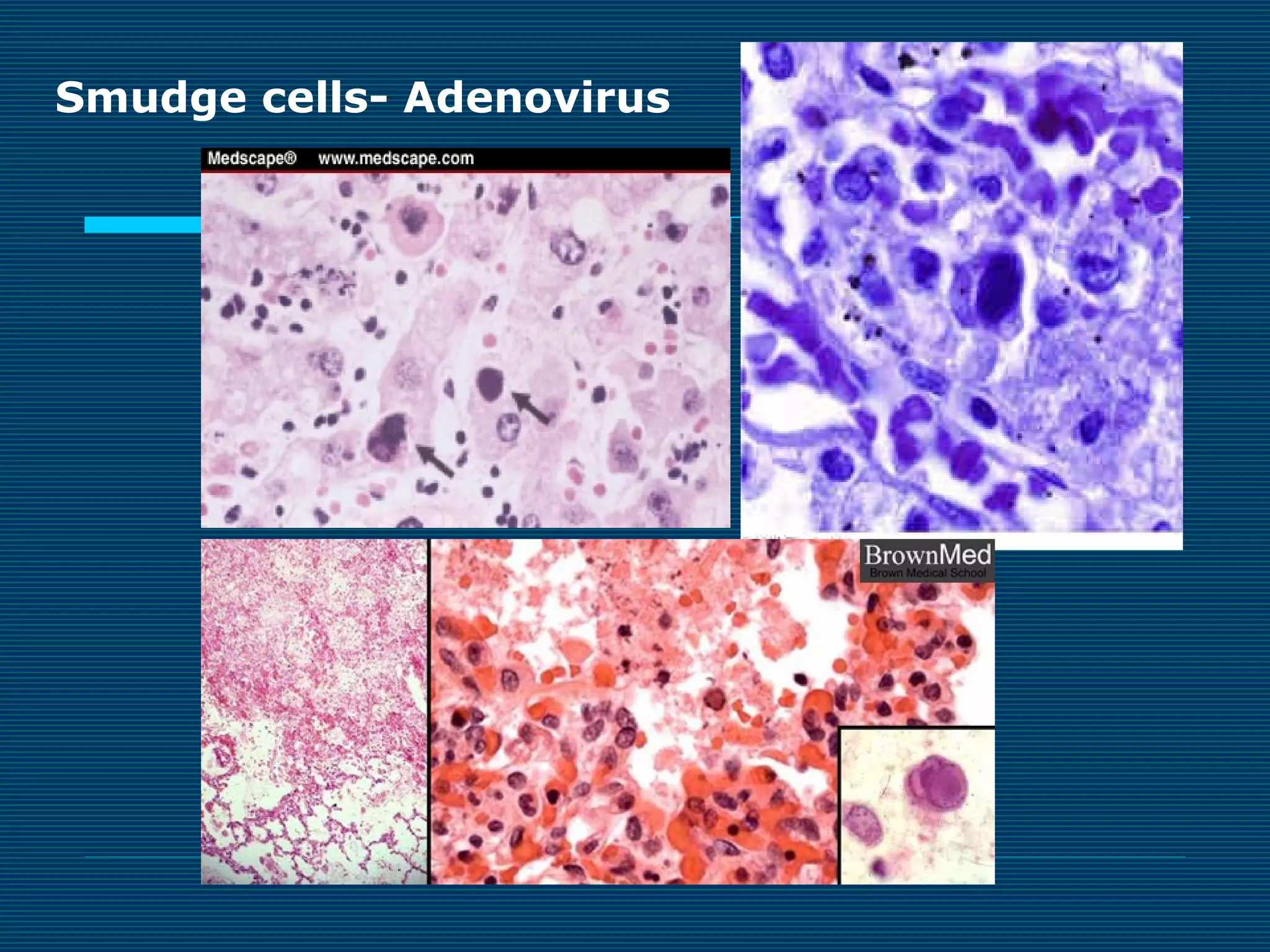
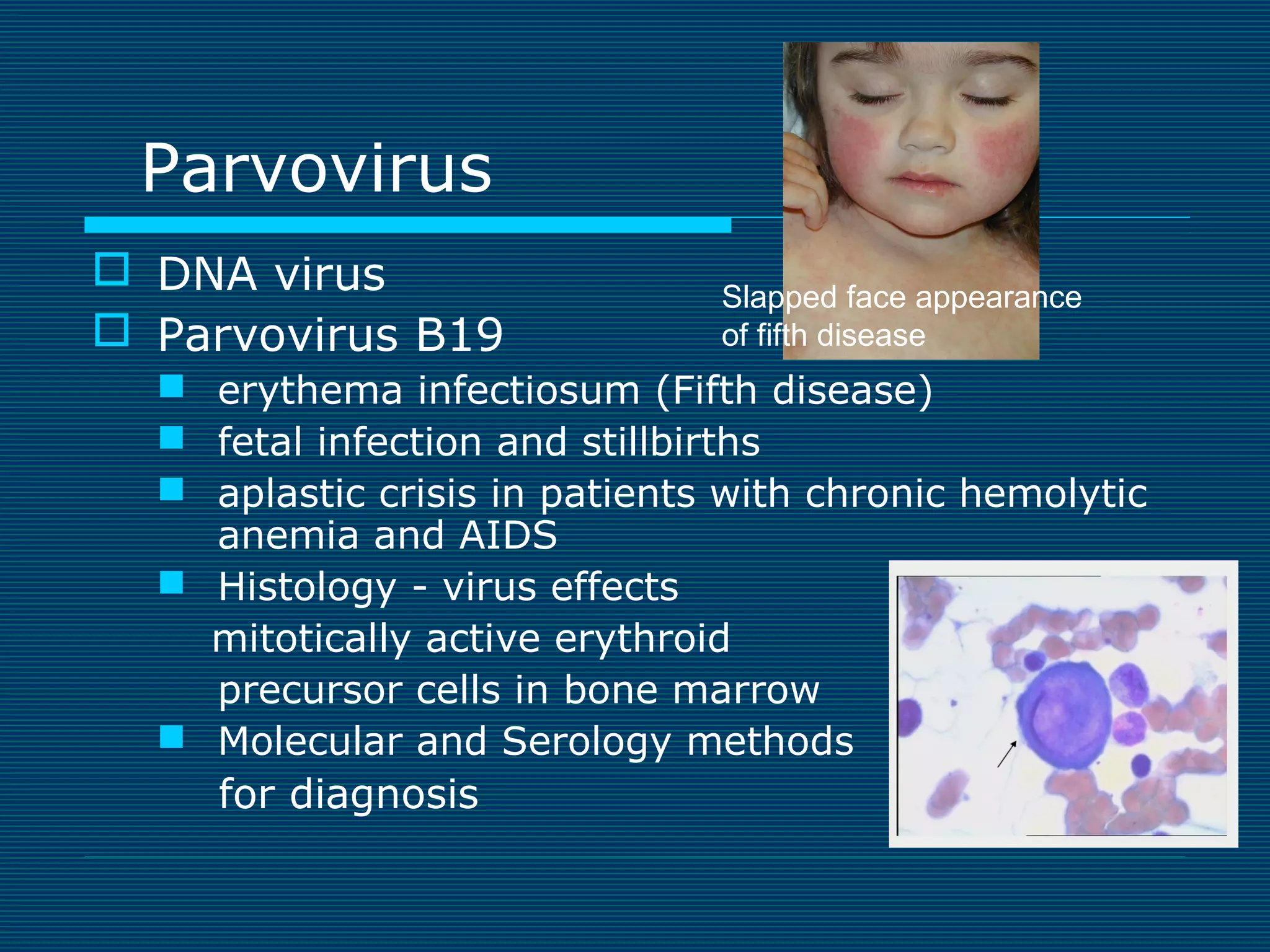
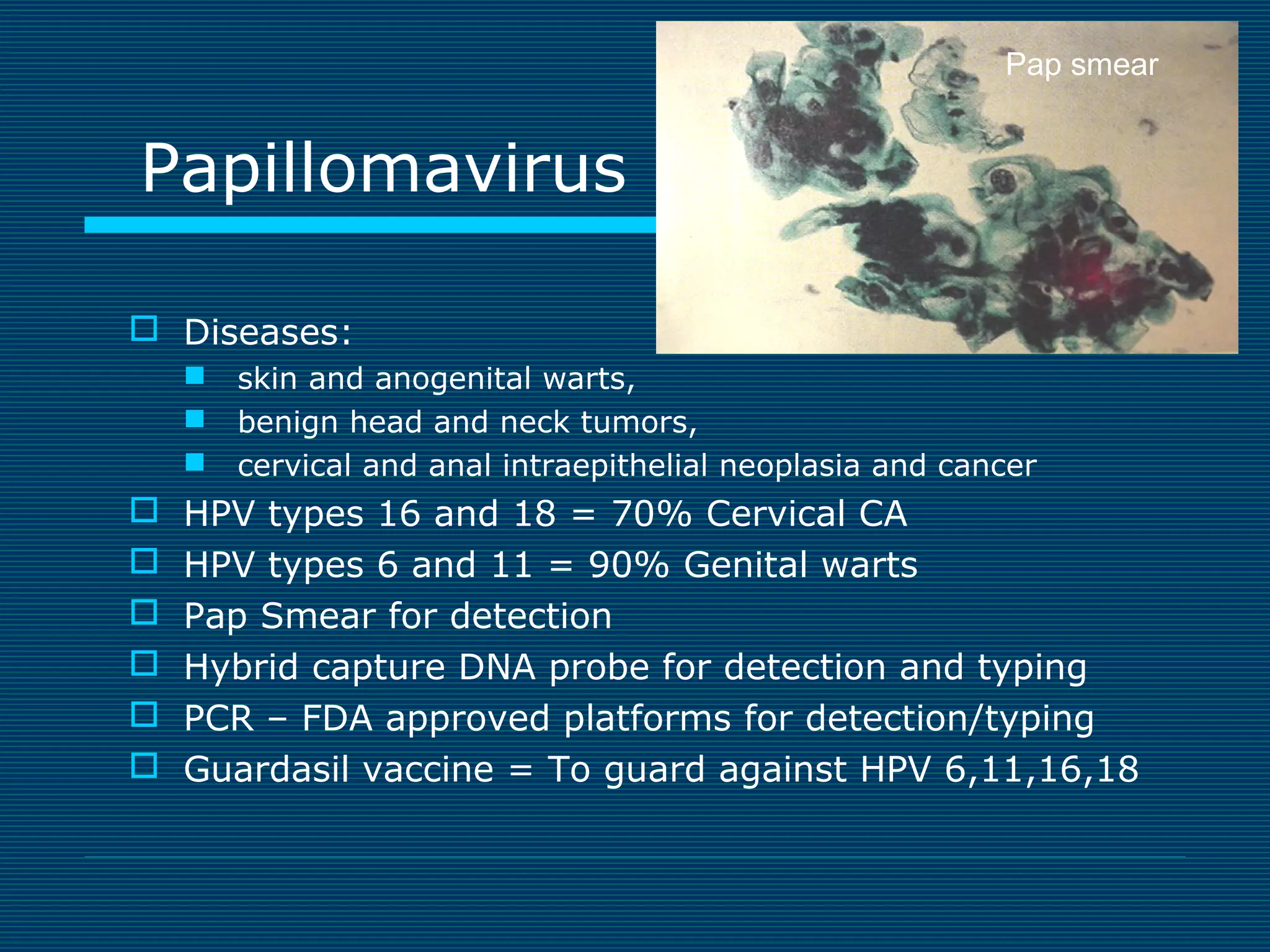
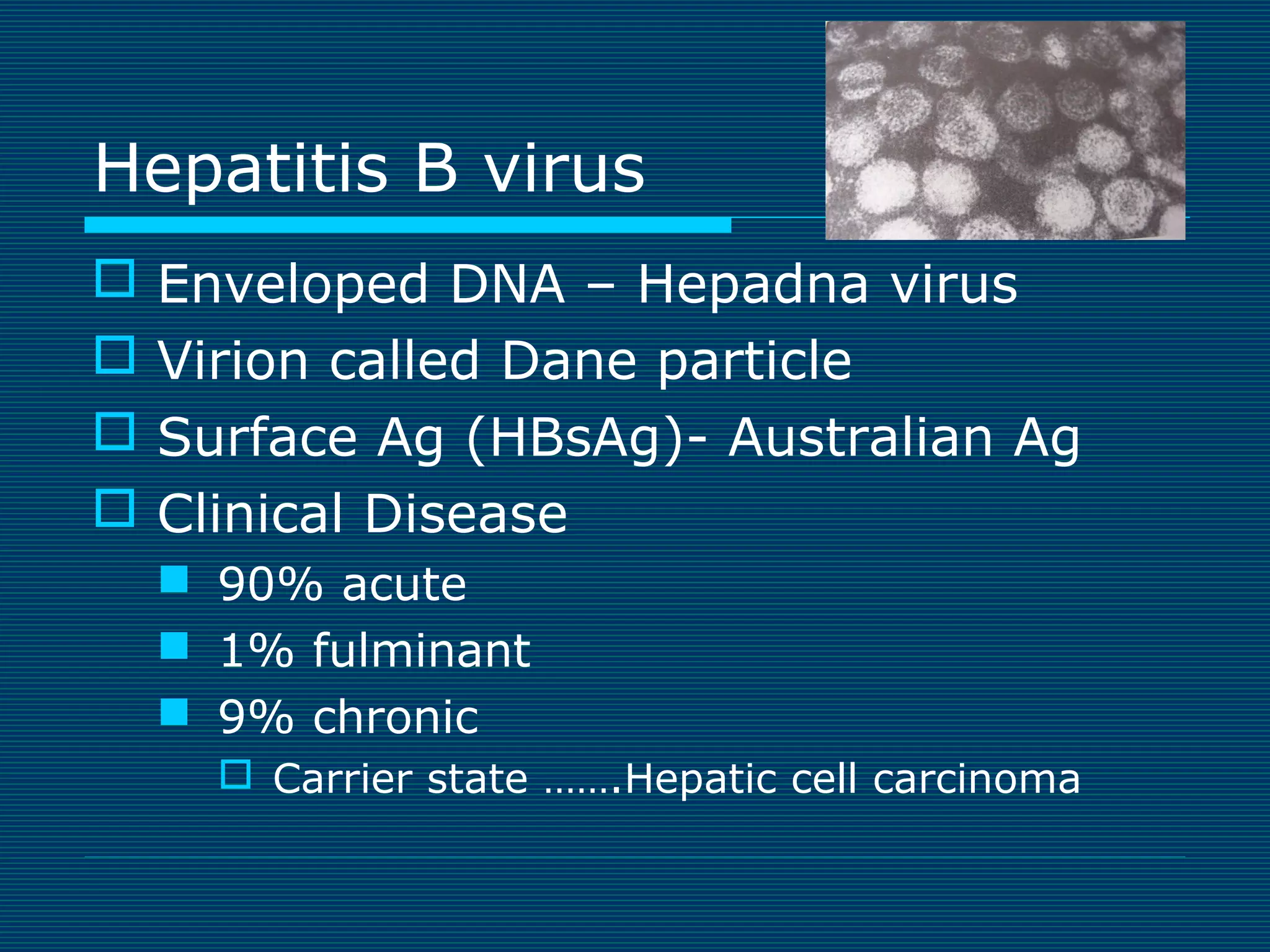
This document provides an overview of diagnostic techniques used in virology laboratories, including direct antigen detection, rapid antigen detection assays, molecular detection methods like PCR, viral cell culture techniques, and specimen collection and transport. It discusses the diagnostic methods for specific virus families like Herpesviridae, Adenoviridae, Parvoviridae, Papovaviridae, Hepadnaviridae, Flaviviridae, Picornaviridae, and Orthomyxoviridae. Key points covered include the use of direct fluorescent antibody staining, enzyme immunoassays, viral culture characteristics and cytopathic effects, histopathology features, and serological assays for various virus identification.











![Human Herpes virus
6, 7 & 8
HH6
Roseola [sixth disease]
6m-2yr high fever & rash
HH7
CMV like Disease
HH8
Kaposi’s sarcoma
Castleman’s disease
Onion skin of
Castleman disease](https://image.slidesharecdn.com/webpagevirology2013-131120230918-phpapp01/75/Virology-Review-24-2048.jpg)



![Polyomavirus
Giant Glial Cells of JCV
JC virus [John Cunningham]
Cause of Progressive multifocal
leukoencephalopathy
Encephalitis of immune
suppressed
Destroys oligodendrocytes
in brain
BK virus
Causes latent virus infection in kidney
Progression due to immune suppression
Hemorrhagic cystitis
Histology/PCR for diagnosis](https://image.slidesharecdn.com/webpagevirology2013-131120230918-phpapp01/75/Virology-Review-33-2048.jpg)






![Influenzae A
Disease: fever, malaise …. death
Diagnosis
Cell culture obsolete [RMK]
Enzyme immunoassay on paper membrane can be
used in outpatient setting – Rapid but low sensitivity
(60%) and can have specificity issues in off season.
Amplification (PCR) gold standard for Influenza
Detection
Treatment: Amantadine and Tamiflu
Seasonal variation in susceptibility
Vaccinate to prevent
Influenza B
Milder form of Influenza like illness
Usually <=10% of cases /year](https://image.slidesharecdn.com/webpagevirology2013-131120230918-phpapp01/75/Virology-Review-47-2048.jpg)
![Measles
Measles
Fever, Rash, Dry Cough, Runny Nose,
Sore throat, inflamed eyes (photosensitive)
Respiratory spread - very contagious
Koplik’s spots – bluish discoloration inner
lining of the cheek
Subacute sclerosing panencephalitis [SSPE]
Rare chronic degenerative neurological disease
Persistent infection with mutated measles virus
due to lack of immune response
Diagnosis: Clinical symptoms and Serology
Vaccinate – MMR (Measles, Mumps, Rubella) vaccine
Treatment: Immune globulin, vitamin A](https://image.slidesharecdn.com/webpagevirology2013-131120230918-phpapp01/75/Virology-Review-49-2048.jpg)