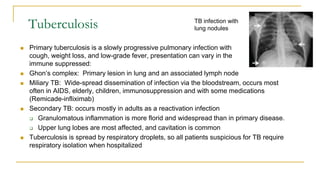
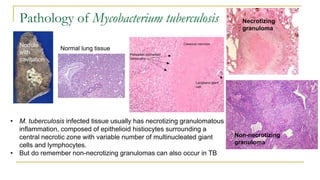
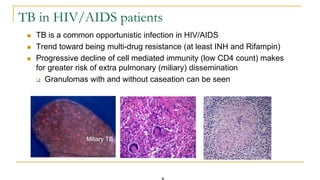
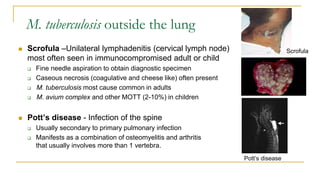
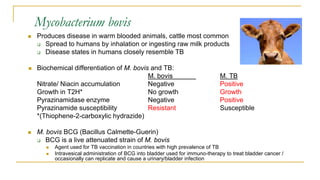
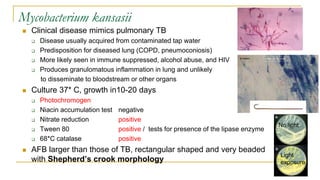
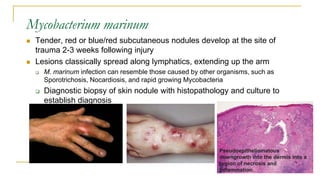
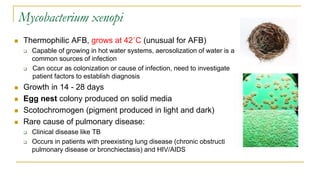
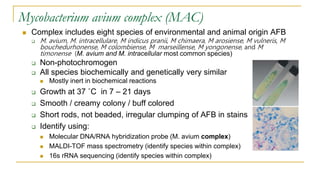
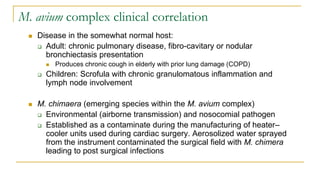
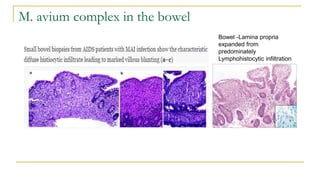
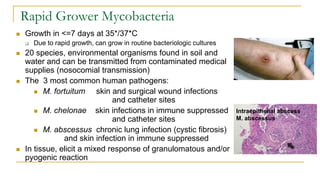
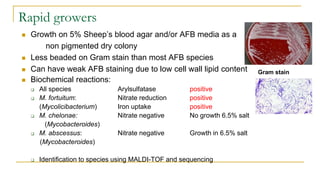
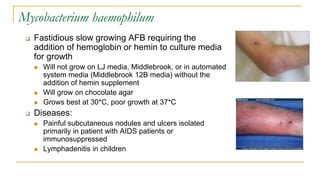
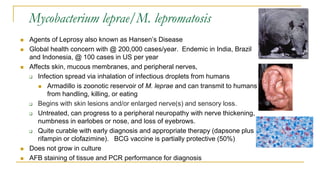
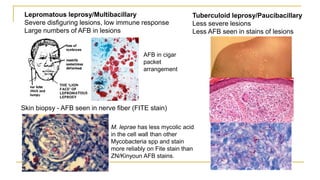
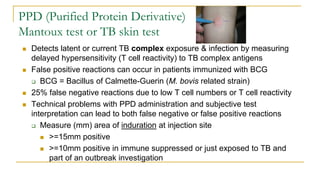
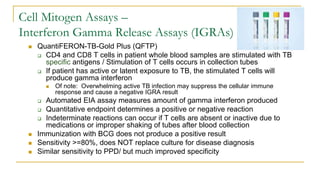
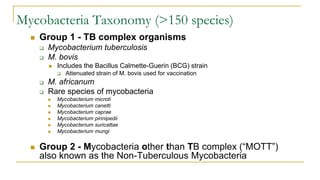
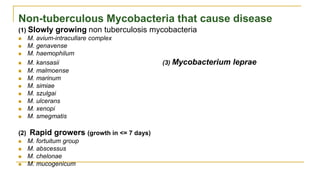
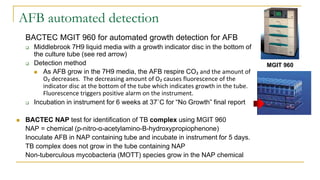
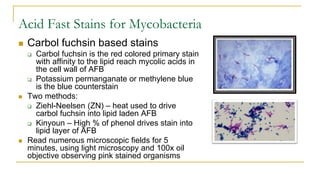
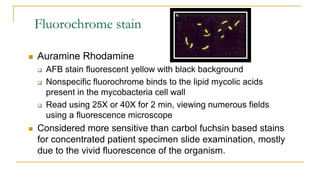
This document provides an overview of mycobacteriology. It discusses acid-fast bacilli (AFB) staining and taxonomy. Laboratory safety level 3 is required for working with mycobacteria. Diagnostic testing includes the tuberculin skin test, interferon-gamma release assays, and molecular detection of TB from respiratory specimens. Culture media like Middlebrook and Lowenstein-Jensen are used to grow mycobacteria. Identification has transitioned to methods like MALDI-TOF mass spectrometry from older biochemical techniques. Common pathogens include Mycobacterium tuberculosis and non-tuberculous mycobacteria.

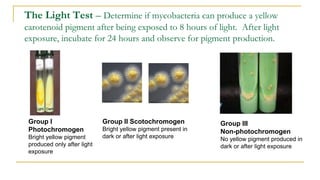
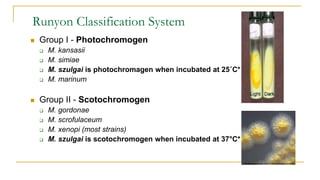
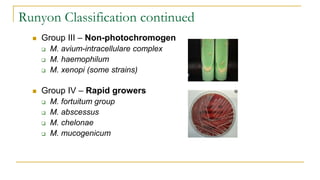



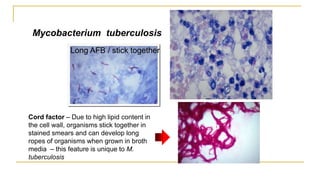
![Acid Fast Mycobacteria morphology
M. avium complex
Short rods tend to randomly
clump.
M. tuberculosis - Organisms
are long, often beaded, and can
appear as if they are sticking
together [due to cord factor]
In broth
cultures -
ropes of AFB
can form due to
cord factor
M. kansasii – AFB are
large, beaded, and tend to
randomly clump, occasional
bent organisms known as
Shepherd’s crook
morphology.](https://image.slidesharecdn.com/mycobacteriologyupdate2023-230120055252-fe25364b/85/Mycobacteriology-Update-2023-23-320.jpg)