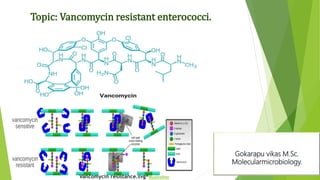
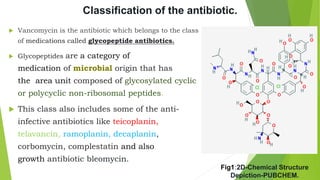
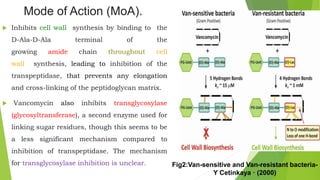
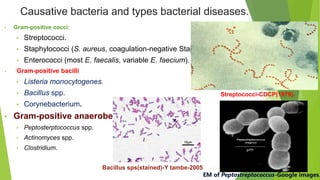
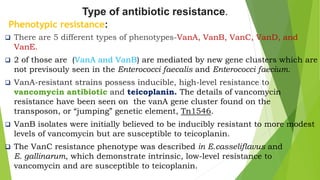
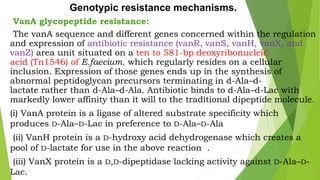
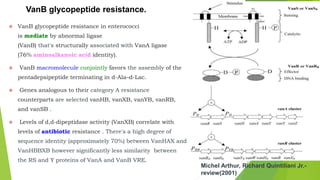
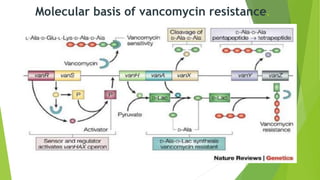
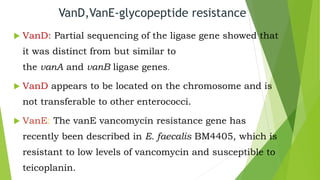
The document discusses vancomycin resistance in enterococci, detailing the classification, mechanism of action, and various resistance phenotypes such as vana, vanb, vanc, vand, and vane. It highlights the prevalence of vancomycin-resistant enterococci (VRE) in various populations, with a specific case study indicating a high carriage rate in Indian pediatric patients. The text underscores the economic burden of antibiotic resistance and emphasizes the urgent need for improved stewardship and stricter regulations to combat this growing public health threat.