This document discusses the principles and methods of voltammetry and polarography. Some key points:
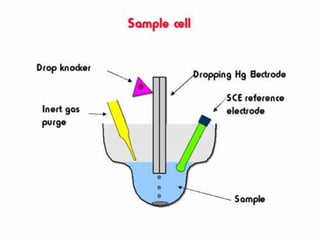
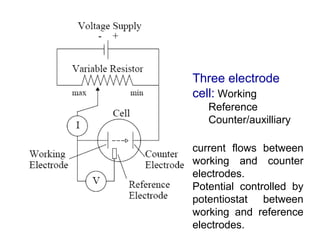
- Voltammetry measures the current-potential curve during electrolysis using a small amount of sample. Polarography uses a dropping mercury electrode as the working electrode.
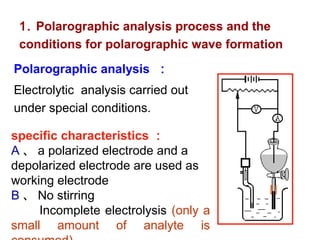
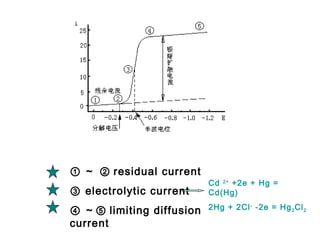
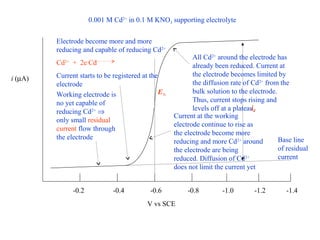
- In polarographic analysis, a polarized working electrode and depolarized reference electrode are used. No stirring is used. Only a small amount of analyte undergoes electrolysis.
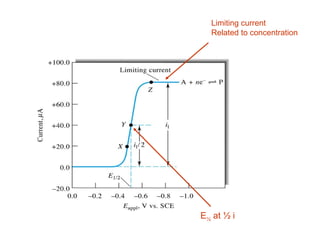
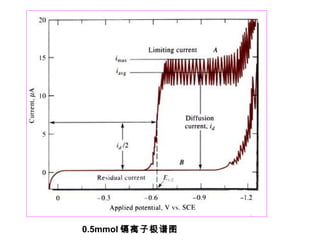
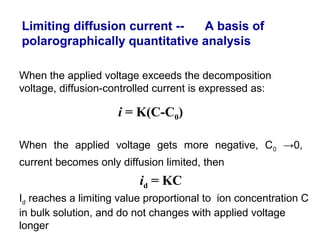
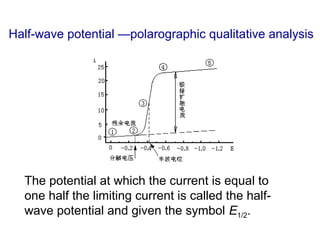
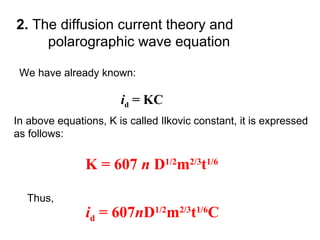
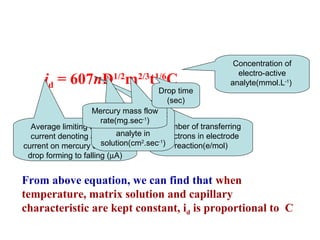
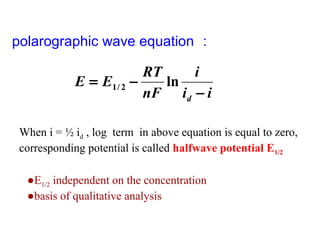
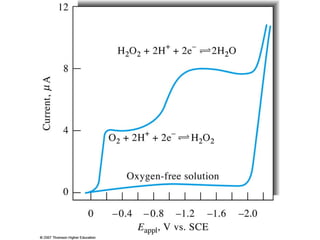
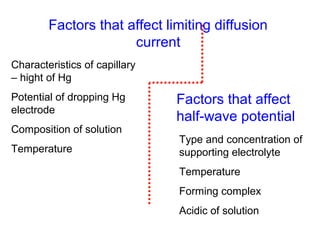
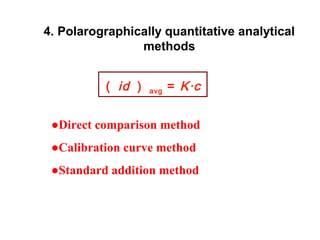
- The limiting diffusion current is proportional to analyte concentration and can be used for quantitative analysis. The half-wave potential is used for qualitative analysis.
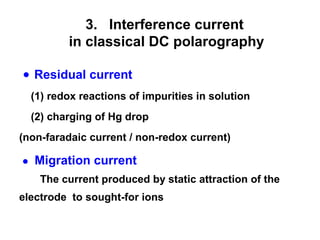
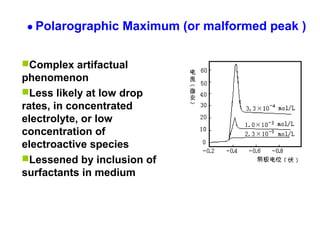
- Factors like temperature, supporting electrolyte composition, and mercury electrode potential affect the limiting diffusion current.