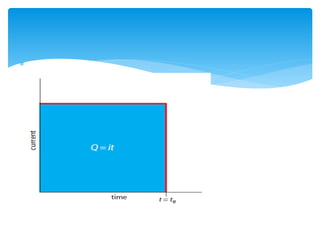
Coulometry is an electroanalytical technique where the amount of electricity (in coulombs) required to complete an electrochemical reaction is measured. There are two main types - potentiostatic coulometry, where the potential is held constant, and coulometric titration with a constant current. The quantity of electricity is directly proportional to the amount of analyte and can be used to determine concentrations. Coulometry has applications in inorganic analysis, analysis of radioactive materials, microanalysis, and determination of organic compounds.




![1] Gasometric coulometers (Hydrogen-oxygen coulometers)
Hydrogen-oxygen coulometer consists of a glass tube of about 50 cm
long and a diameter of 2 cm.
Two platinum sheets of about l.5 sq.cm are joined with a stout
platinum wire serve as the electrodes.
A calibrated tube (gas burette) is connected to the electrolysis tube by
means of a pressure rubber tube and is capable of moving vertically so
as to adjust the pressure of the collected gases to atmospheric
pressure before measuring the volumes of gases.
A 0.5 M solution of potassium sulphate is used as the electrolyte](https://image.slidesharecdn.com/amol21-181107033852/85/coulorometry-13-320.jpg)