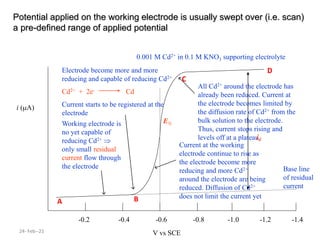
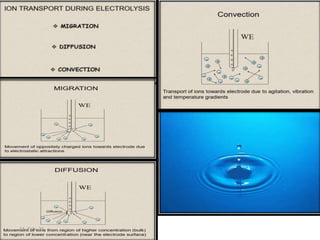
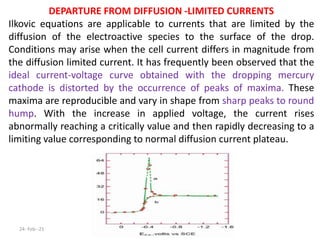
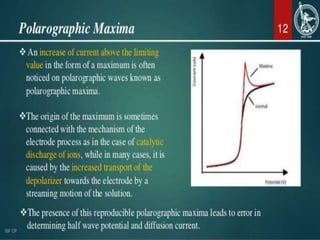
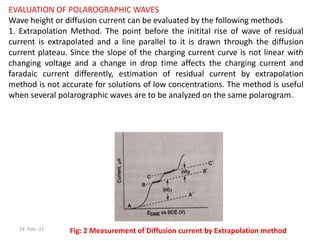
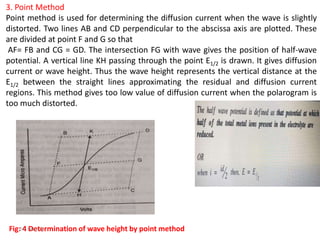
This document discusses polarographic maxima and their interpretation. It begins by explaining how polarography works, involving sweeping the potential of a working electrode and measuring the resulting current. Two types of polarographic maxima are described - first order maxima arising from non-uniform mercury drop polarization, and second order maxima associated with organic compounds that occur over a wider potential range. Methods for removing maxima using suppressors like gelatin are also outlined. The document then discusses factors that can cause non-additive and mixed currents, and provides an example of an anodic wave involving the oxidation of Fe2+. It concludes by describing methods for evaluating diffusion currents from polarographic waves.