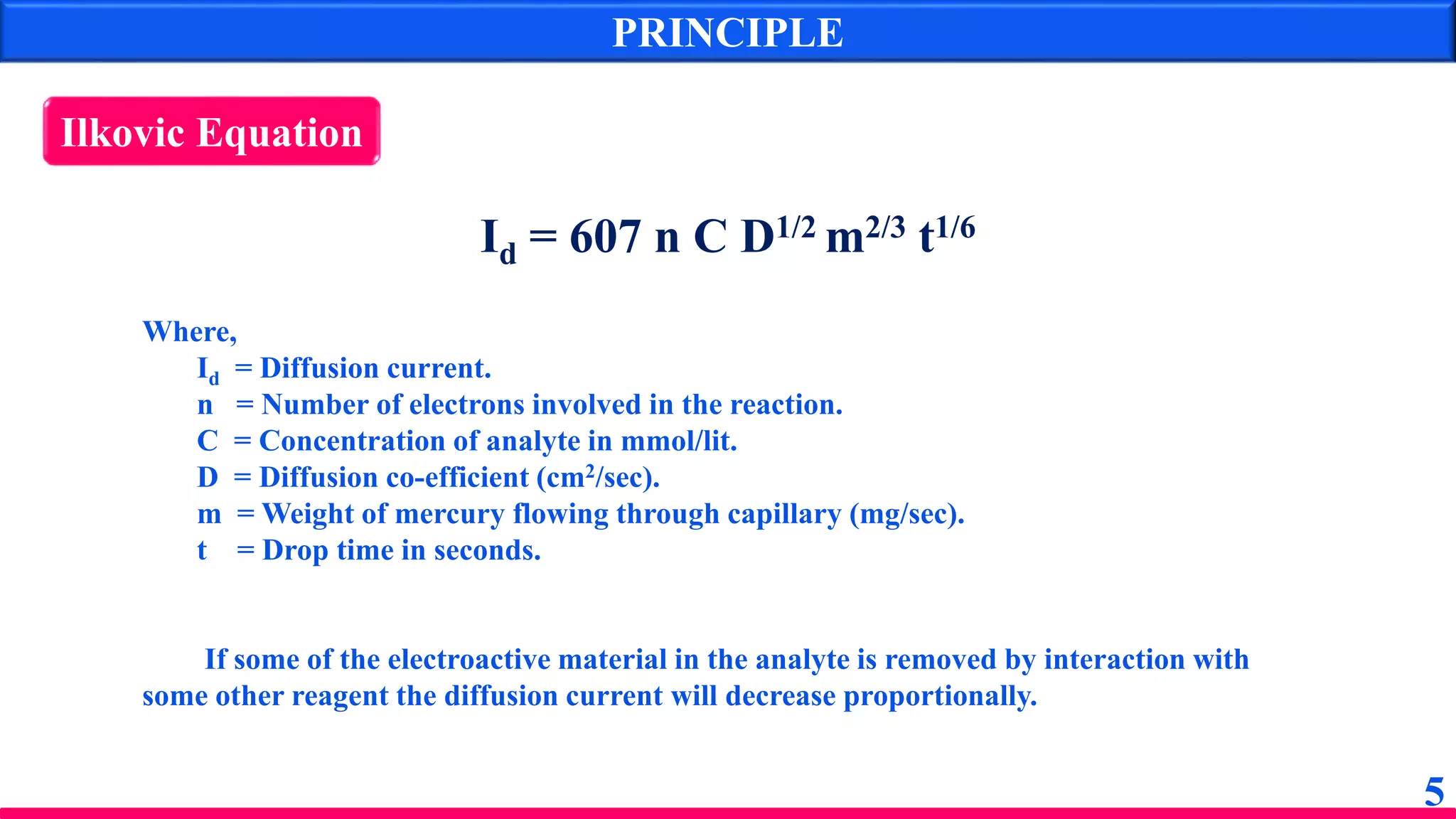
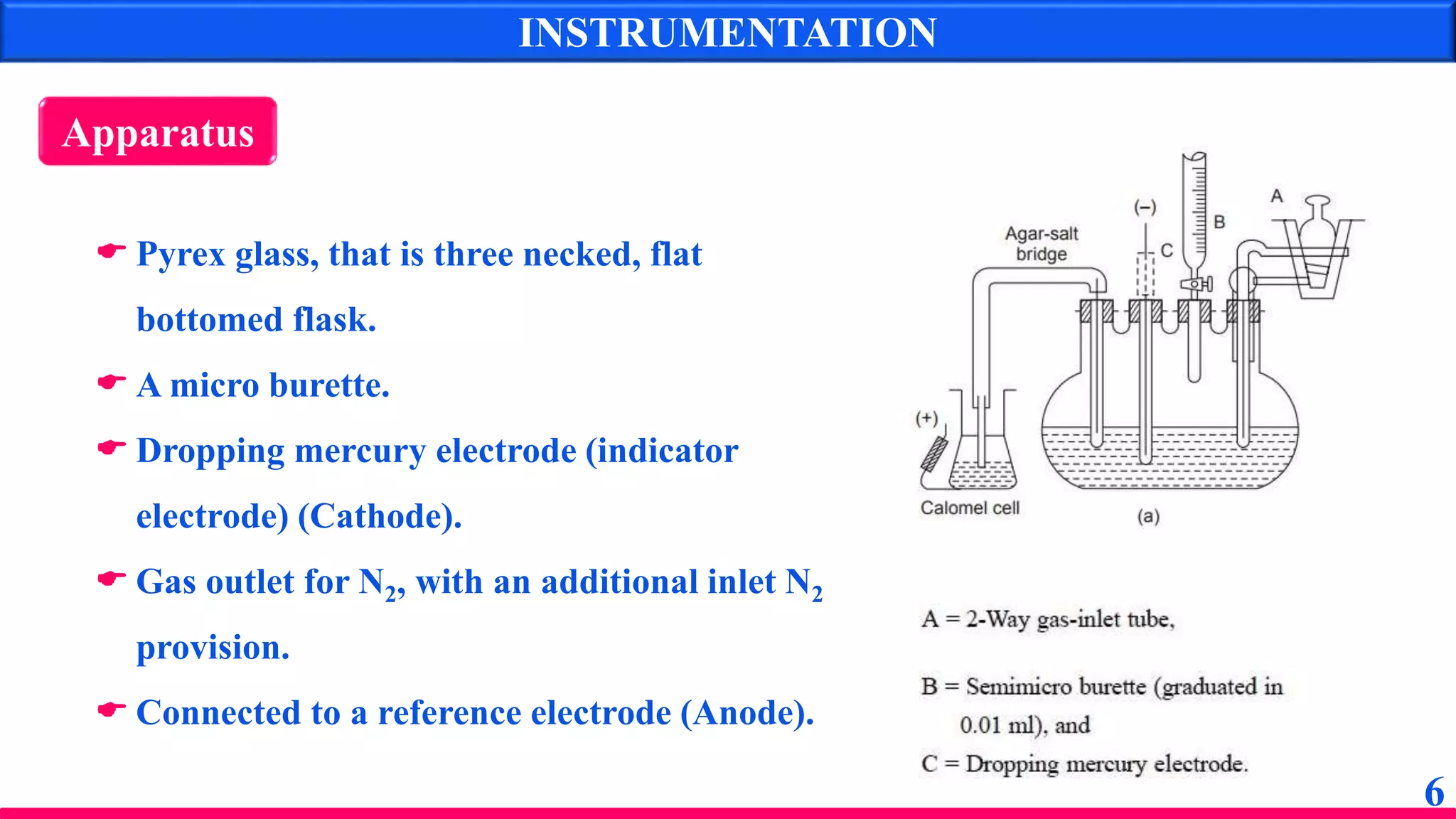
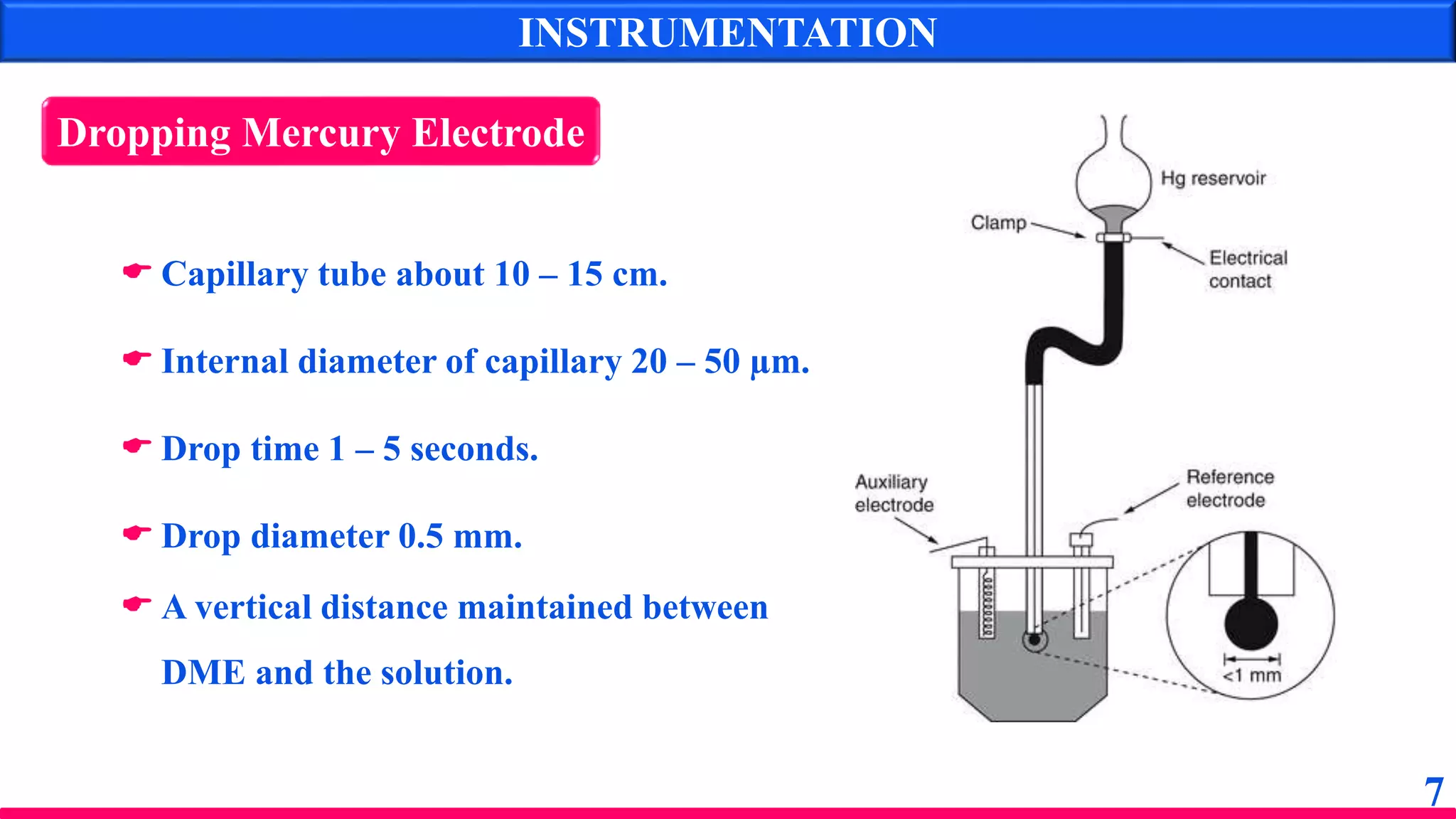
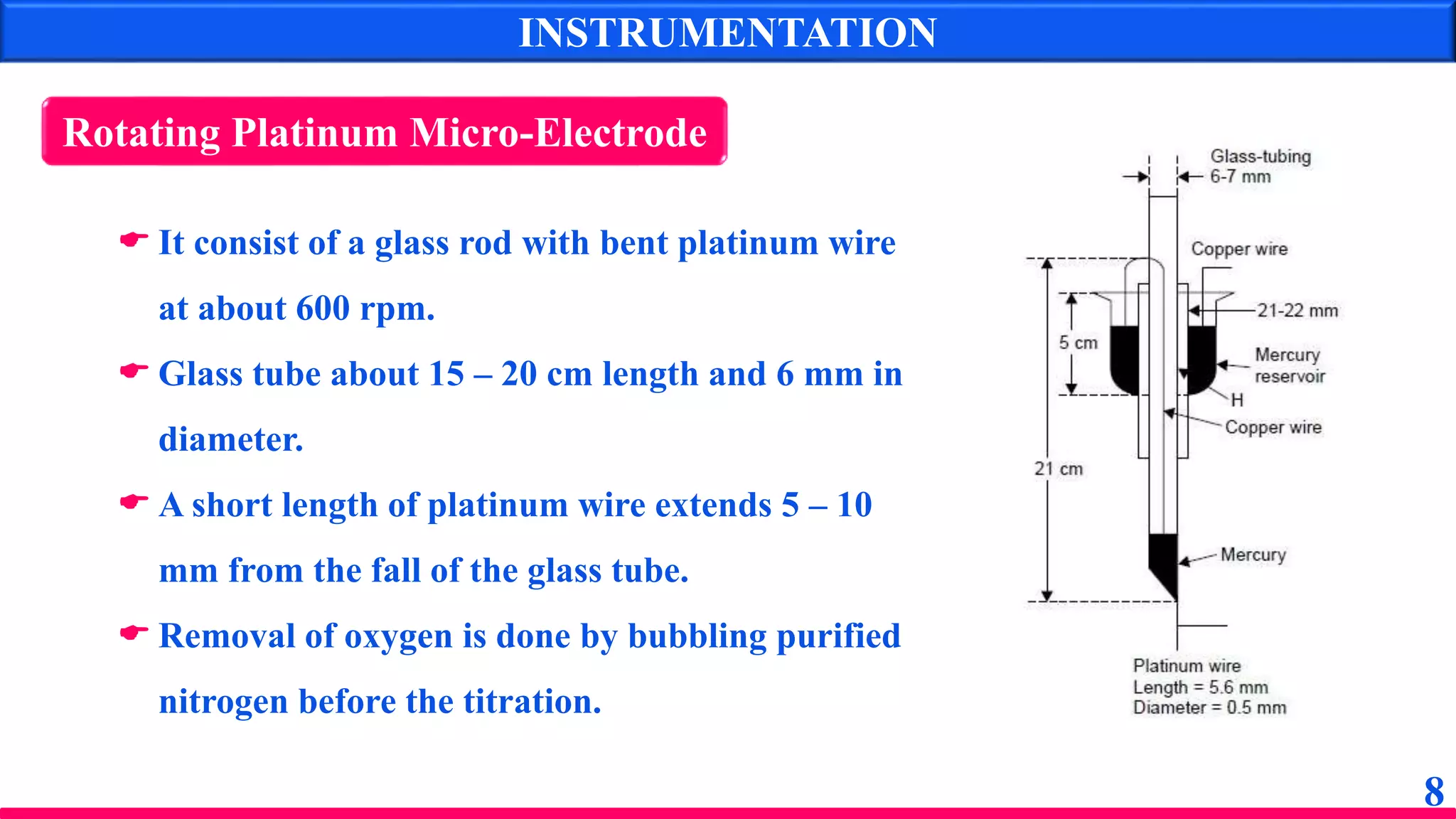
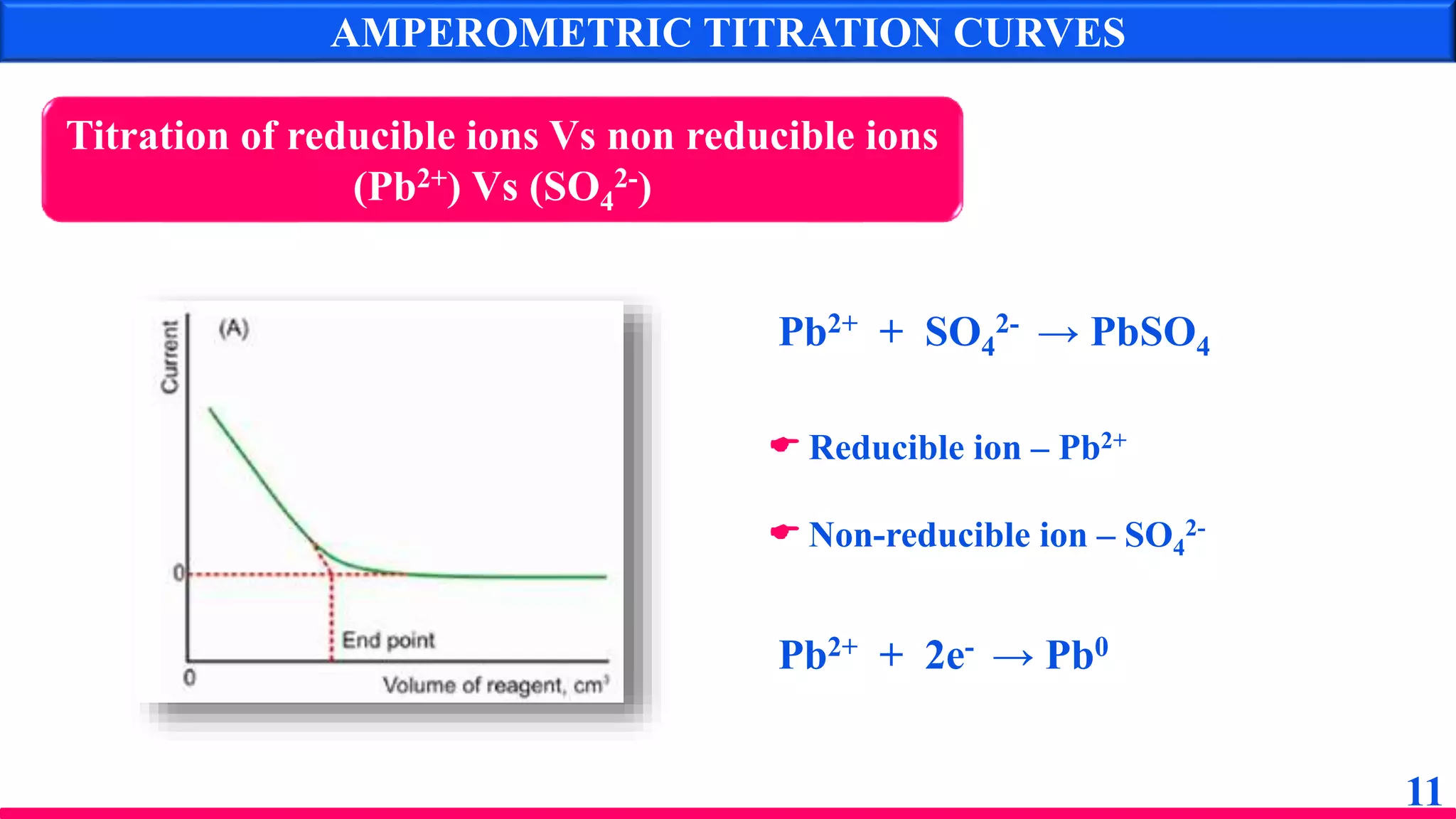
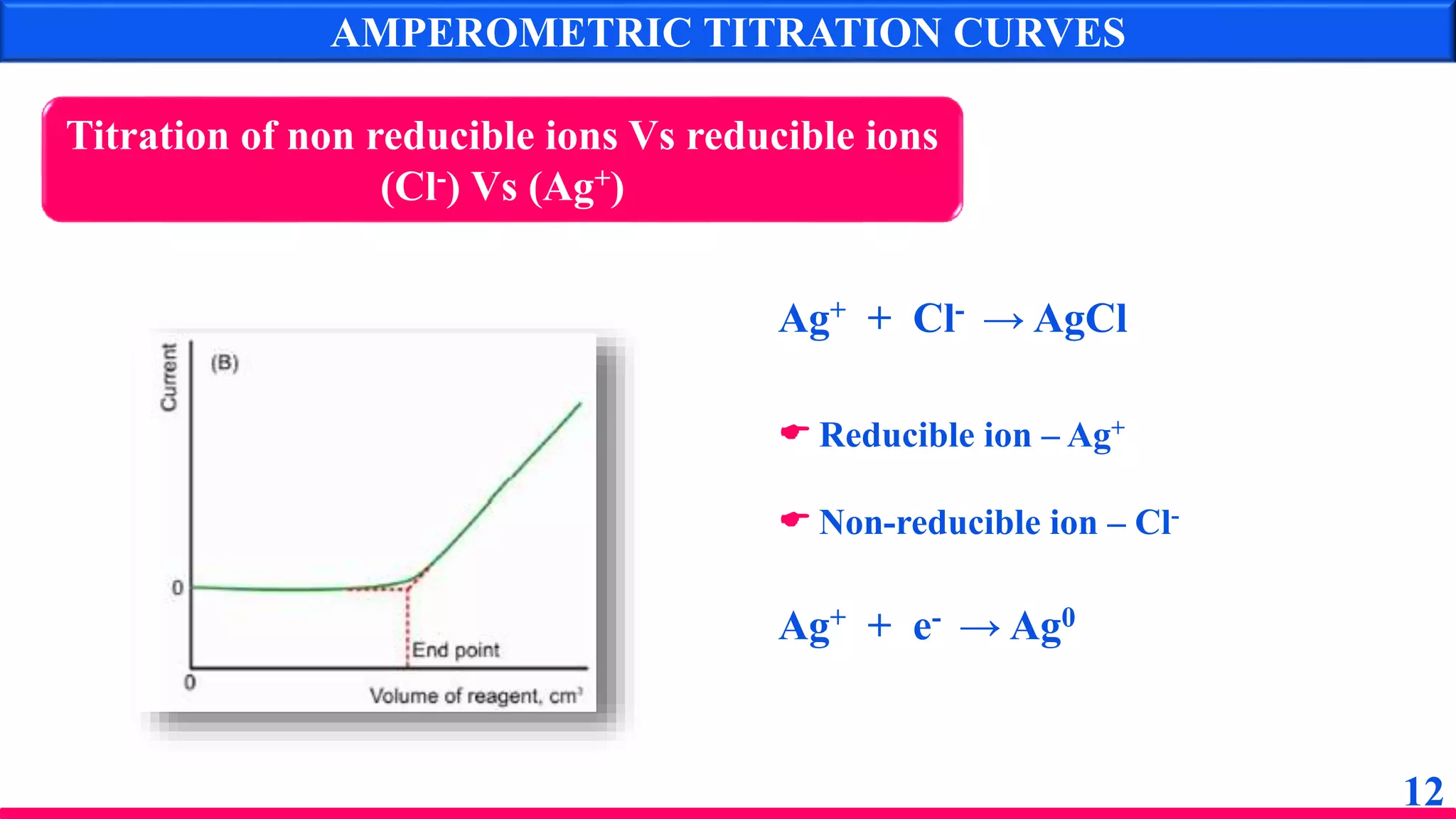
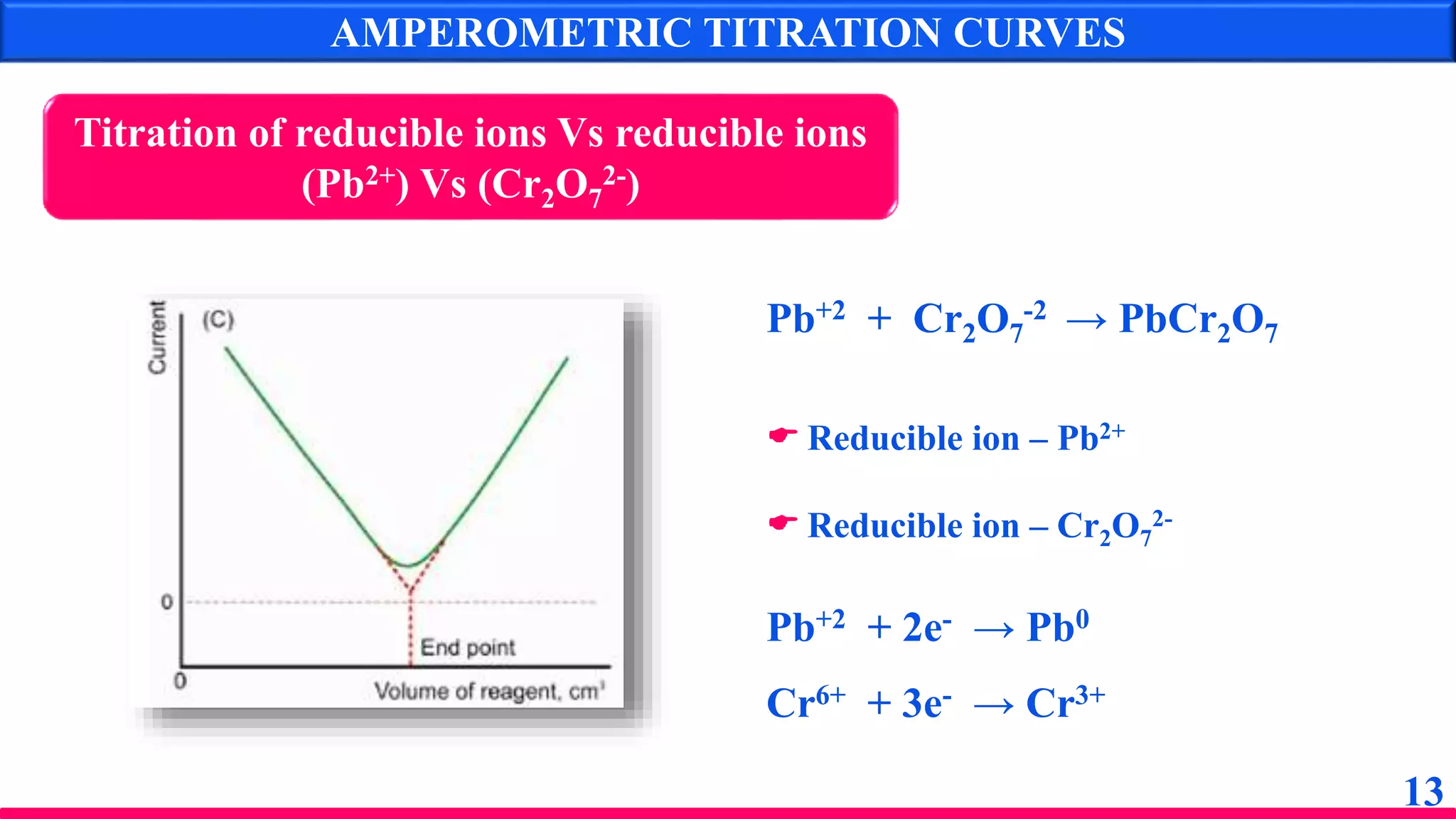
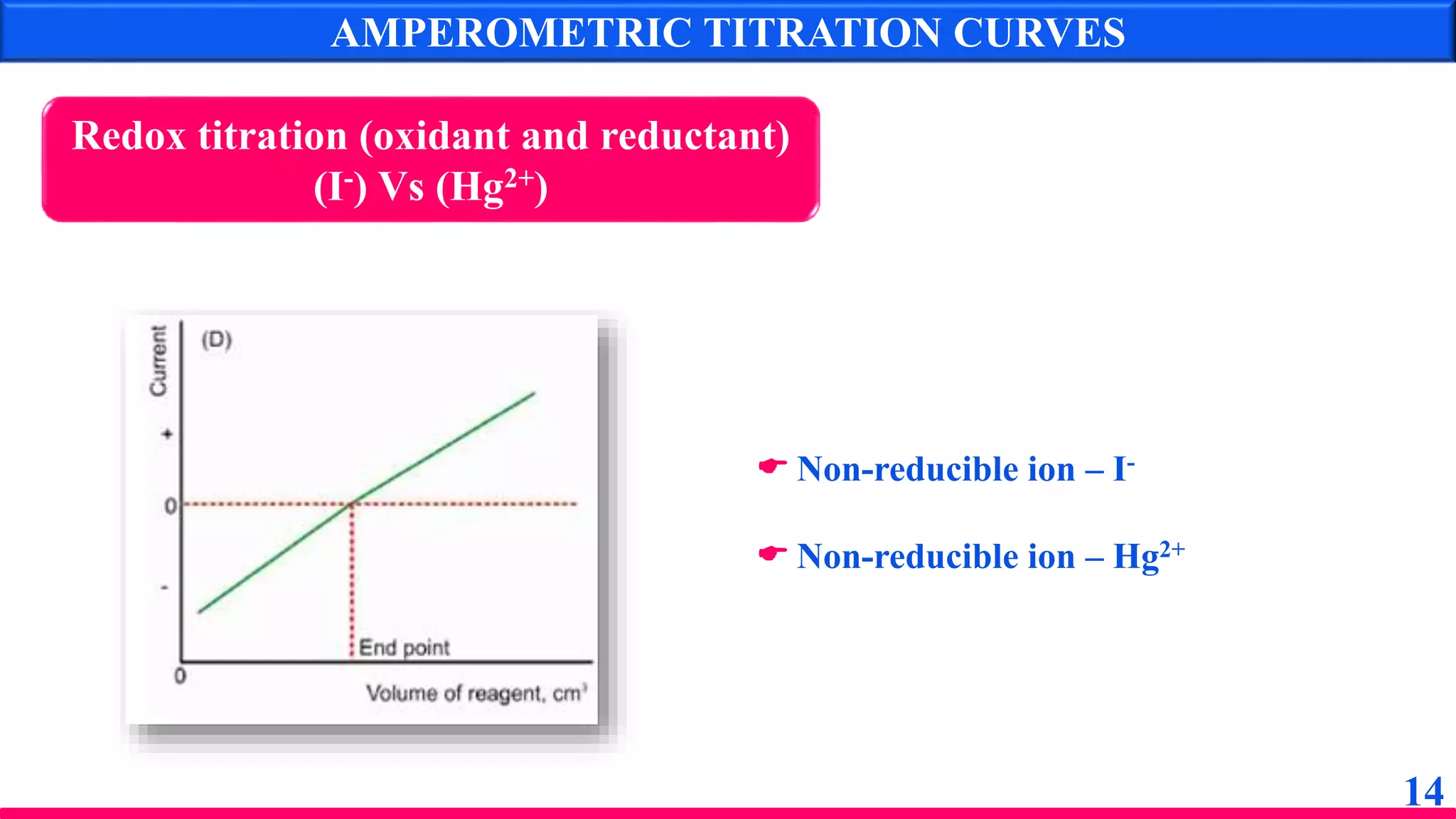
The document discusses amperometry, an electrochemical method for quantitative analysis involving the measurement of current under constant voltage, with applications in various titrations. It explains the principle, instrumentation, advantages, disadvantages, and types of titration curves associated with the technique. Key components include the dropping mercury electrode and rotating platinum micro-electrode, which are used to quantify analyte concentrations based on diffusion current changes.