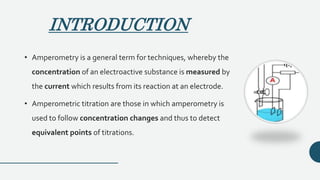
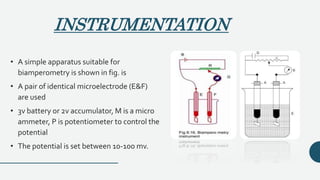
This document discusses amperometry and biamperometry, focusing on their principles, instrumentation, experimental conditions, and procedures for titration, which involves measuring current changes during chemical reactions. Amperometry measures the concentration of electroactive substances via current generated at an electrode, while biamperometry employs two identical microelectrodes and a small applied potential for titration. Both methods offer advantages such as rapid endpoint detection and reduced interference from foreign salts.


![Curves
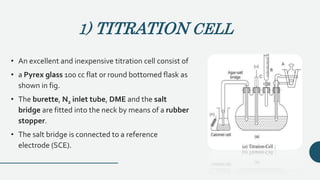
• In fig. A, only the material being titrated is
reduced and gives a diffusion current [For
e.g. Pb [II] ions titrated oxalate or sulphate
ions]
• the current decrease upto the end point.
After the end point the current is fairly
constant.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-11-320.jpg)
![Continue
• In fig. B, Reagent is reduced, analyte is
not.
• [e.g. sulphate ions titrated with Ba [II] or
Pb [II] ions].
• Thus current is constant upto the end
point but rises steadily after the end
point.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-12-320.jpg)
![Continue..
• In fig. C, both analyte and the titrating
reagent are reduced and give diffusion
currents and a sharpV shaped curve is
obtained.
• [for e.g. Pb ions titrated with dichromate ions,
Ni [II] with dimethyl glyoxime]
• Thus current decreases upto the end point &
then rises steadily after the end point.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-13-320.jpg)
![ADVANTAGES
• The titration can usually be avoided out rapidly, since the end point is found
graphically.
• can be carried out at dilutions[10-4M] at which visual or potentiometric
titrations no longer yield accurate results.
• Foreign salts may frequently be present without interference, usually added
as the supporting electrolyte in order to eliminate migration current.
• The results of the titrations are independent of the characteristic of capillary.
• temperature need not be known, it is kept constant during the titration.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-14-320.jpg)

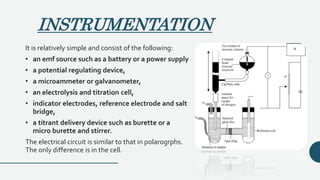
![INTRODUCTION
• These is a modification of the amperometric method.
• In this method, two identical stationary microelectrodes, immersed
in a well stirred solution of the sample are used, instead of the SCE
and the DME.
• A small potential [say 0.01 to 0.1v] is applied between the two
electrodes
• Reversible oxidation to reduction system be present either before
or after the end point.
• also sometimes called as the dead stop end point technique.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-16-320.jpg)
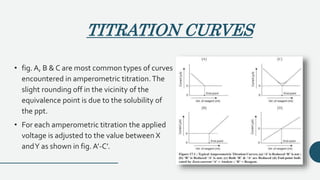
![TITRATION CURVES
• fig. a,b,c the three types of titration curves that are commonly encountered.
• Curve in fig. a is observed when both the reactants are reversible w.r.t. the electrode curves.
• in fig. b & c are obtained when only one of the reactant exhibits reversible behavior.
• The titration of Fe [II] with Ce [IV] is an e.g. of fully reversible system.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-18-320.jpg)
![APPLICATIONS
• Bimperometric titration method has been widely applied to titrations
involving I2, it is also useful with reagents such as Br2,Ti [III], Ce [IV].
• An important use is the titration of H2O with KFR.
• The principle advantage of the method is its simplicity. No reference
electrode is require & the only instrument needed is a simple voltage
divider.](https://image.slidesharecdn.com/pptampero-200906063513/85/Amperometry-and-Biamperometry-19-320.jpg)
