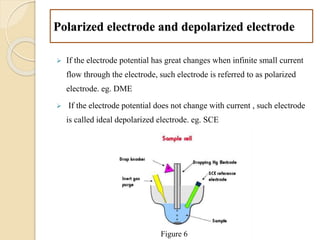
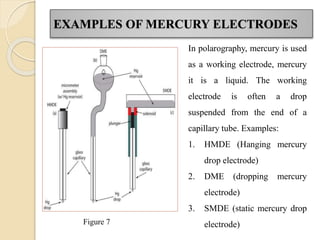
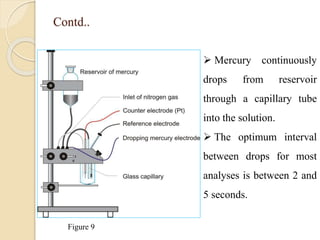
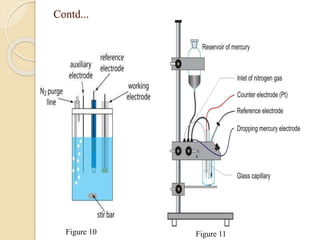
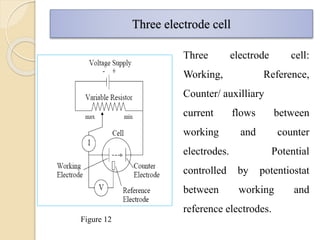
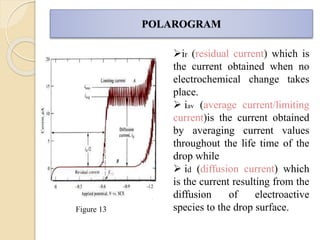
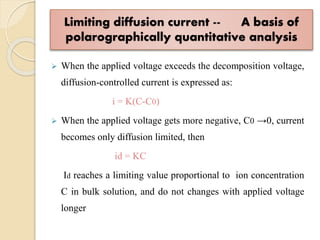
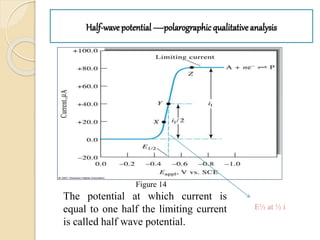
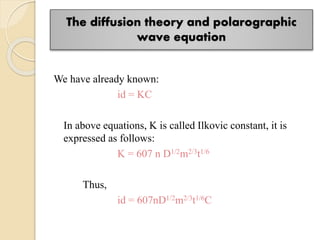
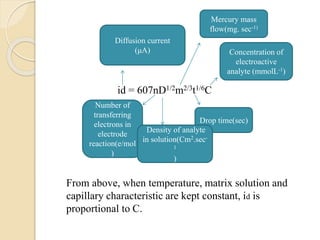
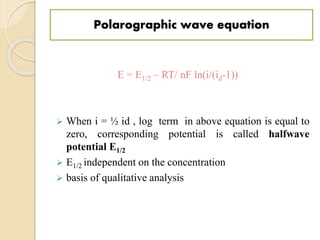
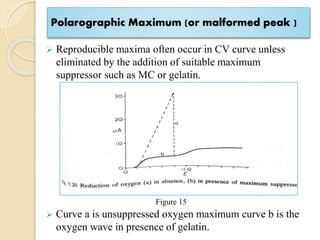
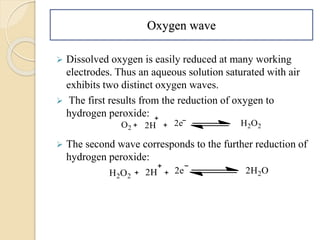
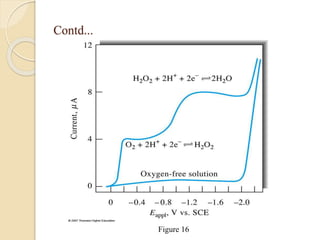
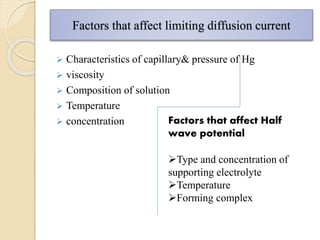
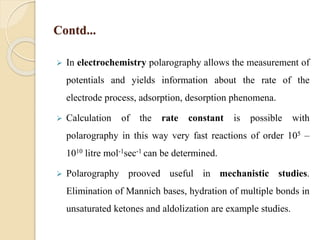
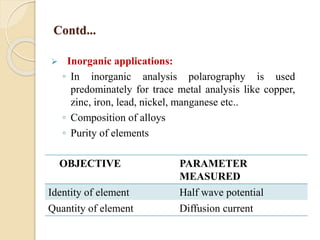
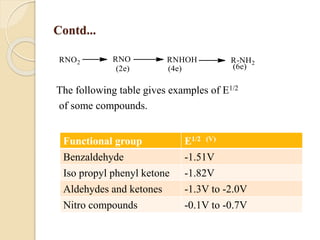
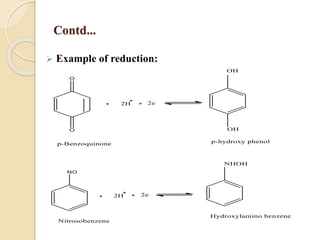
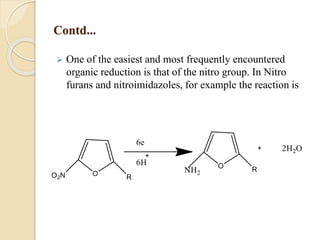
Polarography is an electroanalytical technique that uses a dropping mercury electrode to determine the concentration and nature of substances in a solution. It involves measuring the current between two electrodes - a polarized indicator electrode made of mercury, and a non-polarized reference electrode - as the voltage is gradually increased. The current readings form a polarogram curve that can identify substances based on their half-wave potential and determine concentrations from the limiting diffusion current. Polarography finds applications in fields like water quality testing, medicine, and electrochemistry.