This document discusses electrogravimetry, which is the quantitative analysis of substances by electrolysis. It defines key terms used in electrogravimetry like cathode, anode, current density, and overpotential. It explains Faraday's laws of electrolysis and how they relate to the amount of material deposited. It also describes how controlling variables like cathode potential can be used to selectively deposit metals and separate them from each other.











![21
• The ideal deposit for analytical purposes is adherent, dense, and smooth; in this form it is
readily washed without loss.
• Flaky, spongy, powdery, or granular deposits adhere only loosely to the electrode, and for this
and other reasons should be avoided.
• As a rule, more satisfactory deposits are obtained when the metal is deposited from a solution
in which it is present as complex ions rather than as simple ions.
• Thus silver is obtained in a more adherent form from a solution containing the [Ag(CN z )] -
ion than from silver nitrate solution.
• Nickel when deposited from solutions containing the complex ion [Ni(NH3)6] 2+ is in a very
satisfactory state for drying and weighing.
• Mechanical stirring often improves the character of the deposit, since large changes of
concentration at the electrode are reduced, i.e. concentration polarisation is brought to a
minimum
• Increased current density up to a certain critical value leads to a diminution of grain size of the
deposit
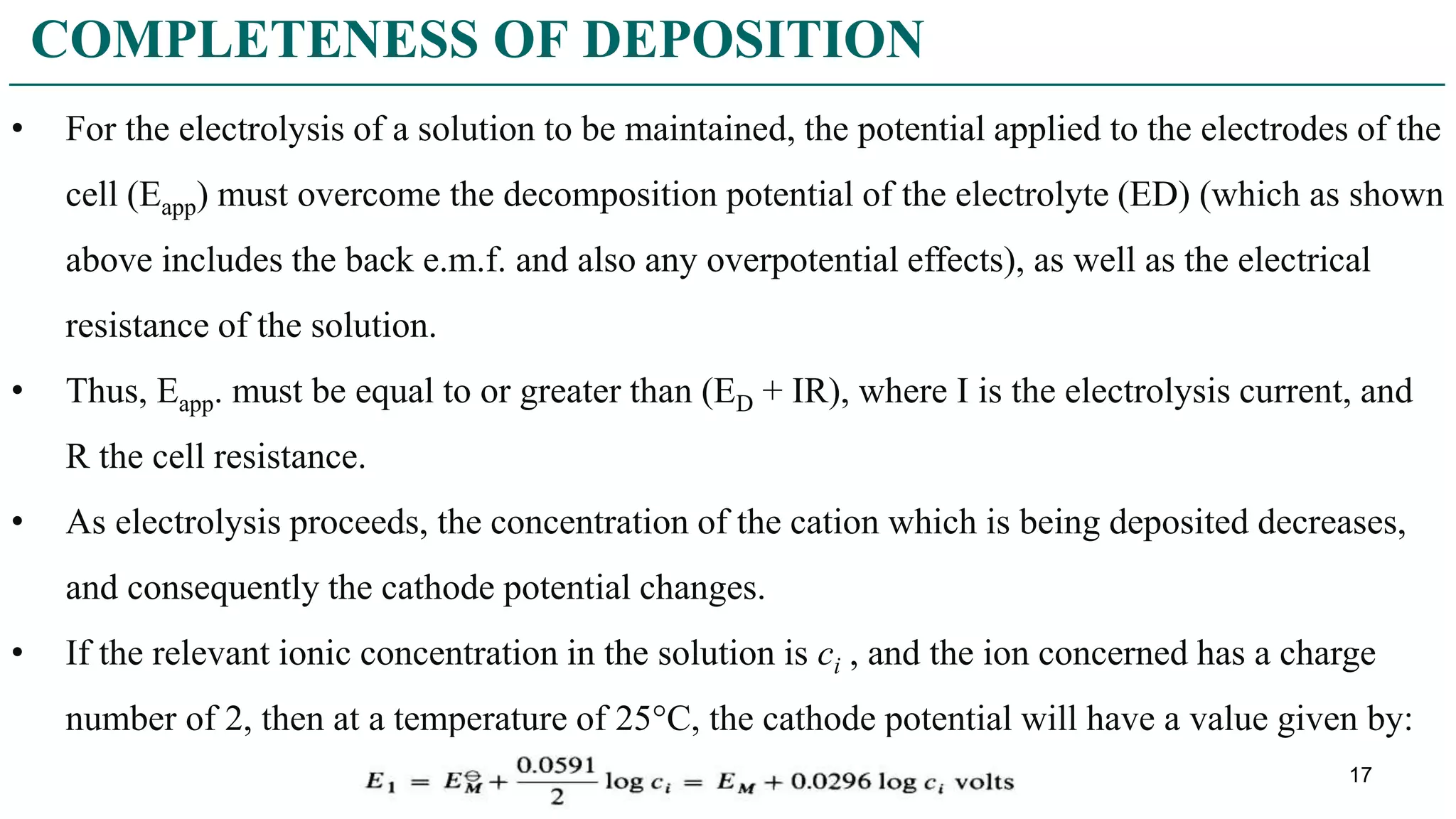
CHARACTER OF THE DEPOSIT](https://image.slidesharecdn.com/electrogravimetry-211216084524/75/Electrogravimetry-21-2048.jpg)
