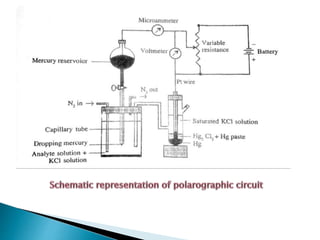
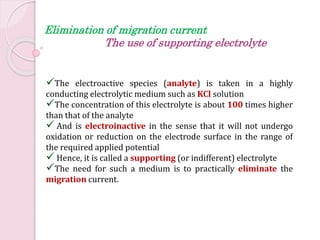
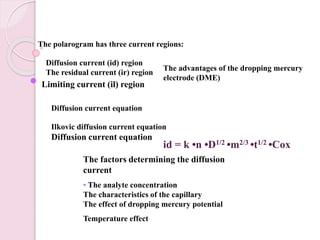
Polarography uses a dropping mercury electrode (DME) to measure the current flowing through an electrochemical cell as a function of the applied potential. A polarogram plots this current versus potential and provides qualitative and quantitative information about species undergoing oxidation or reduction reactions. Jaroslav Heyrovsky invented the polarographic method in 1922 and won the Nobel Prize for his contributions to electroanalytical chemistry. All modern voltammetric methods originate from polarography. The DME provides advantages like a reproducible surface area and the ability to form amalgams with metal ions.