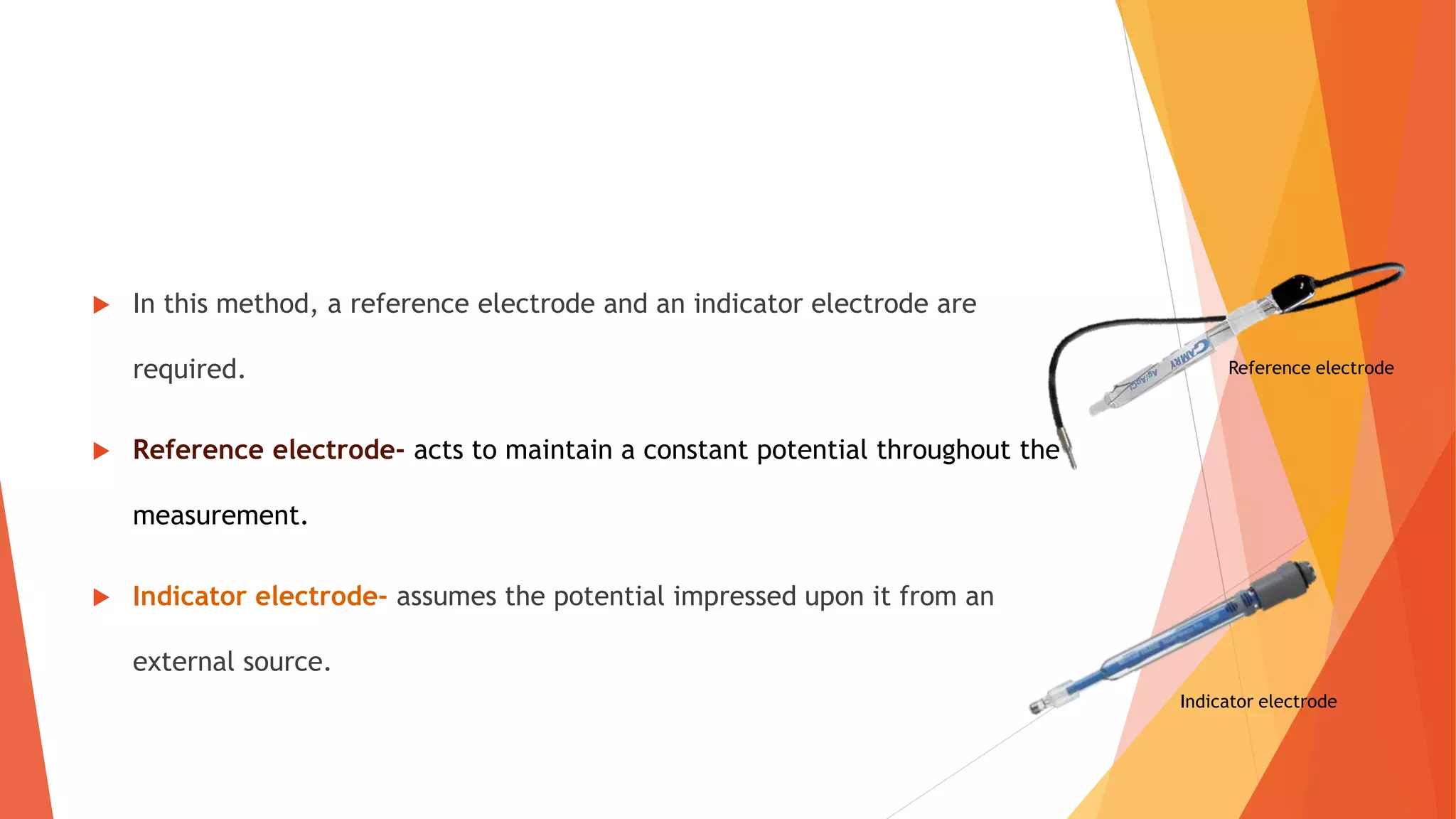
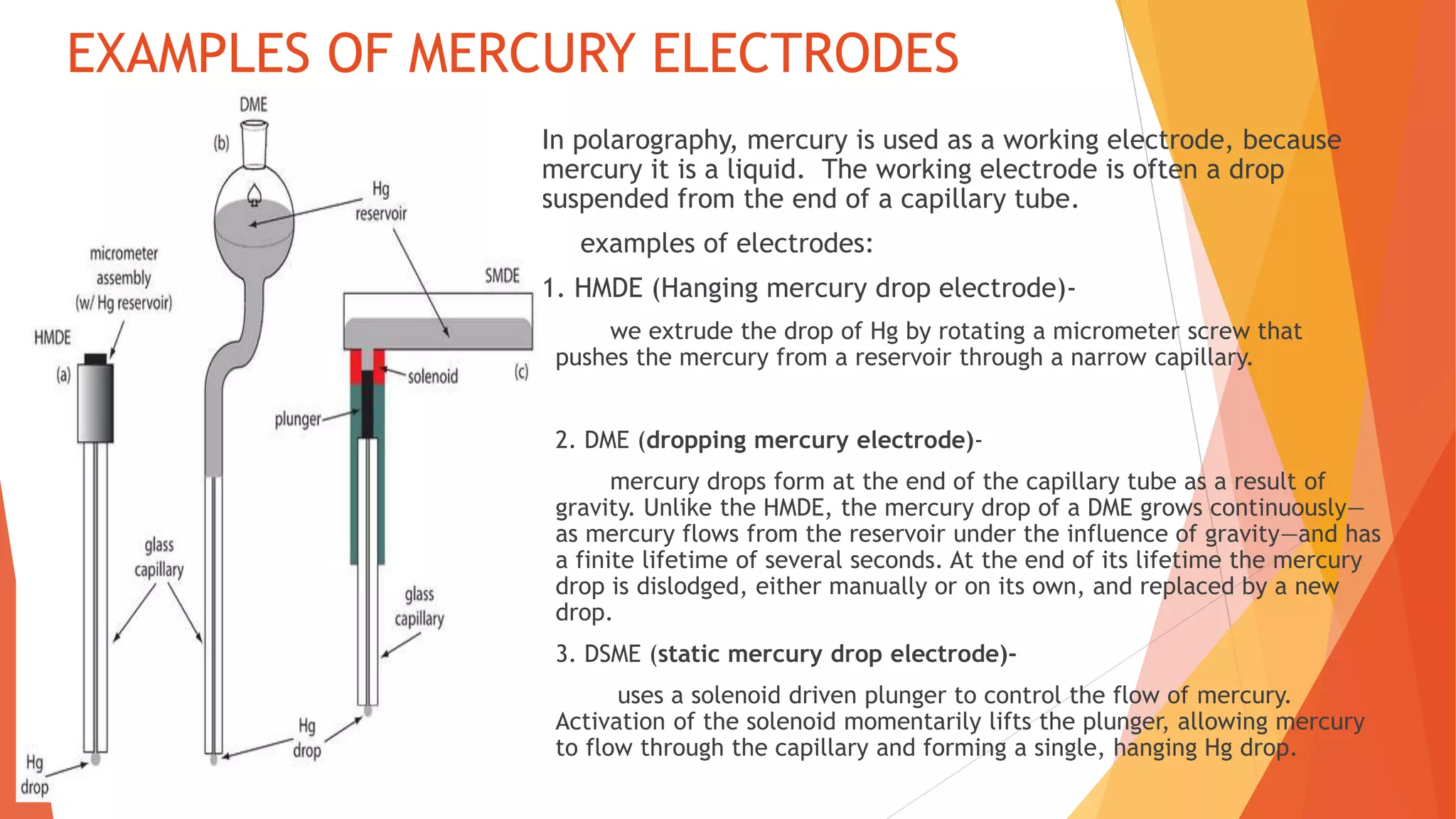
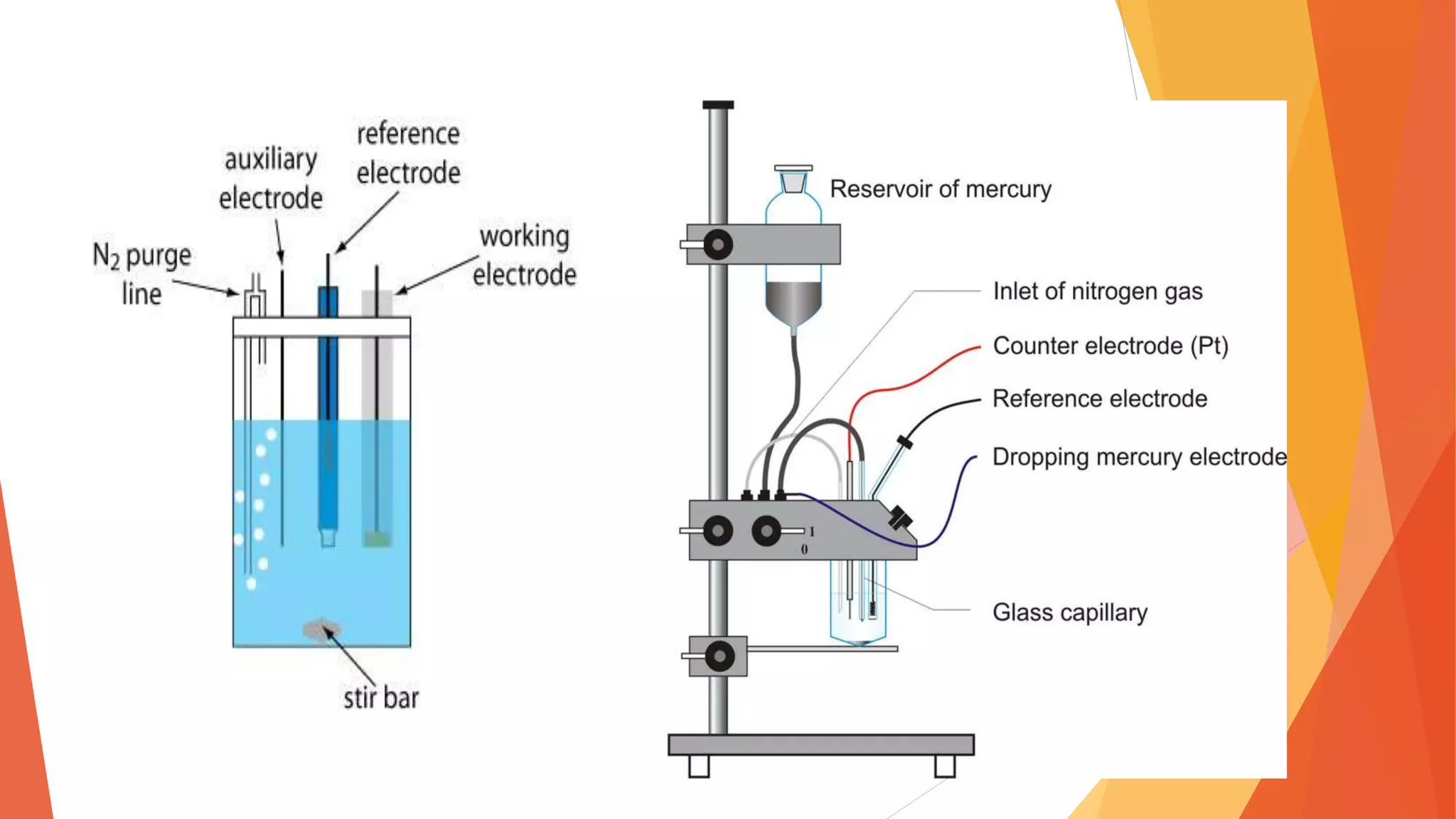
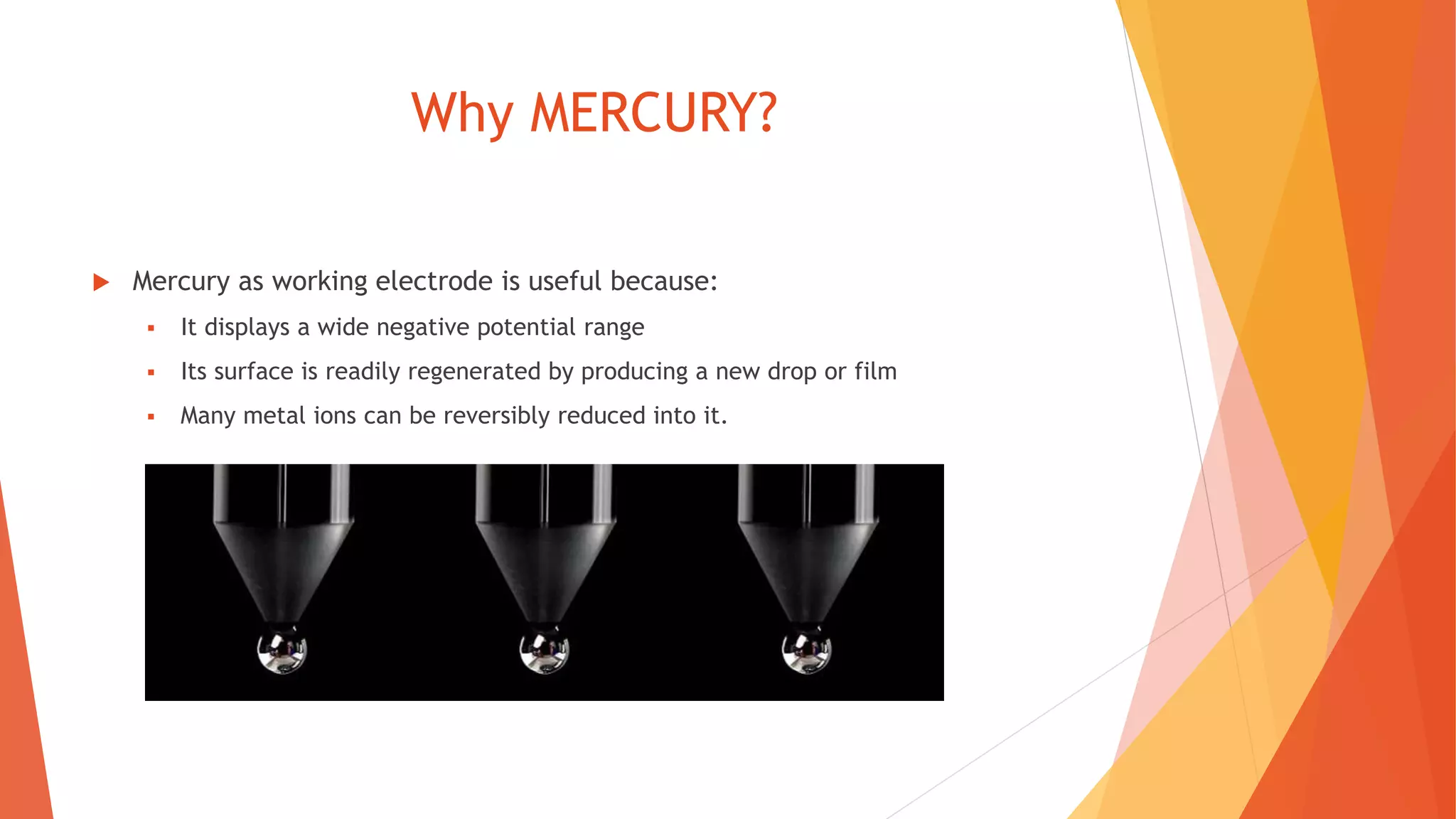
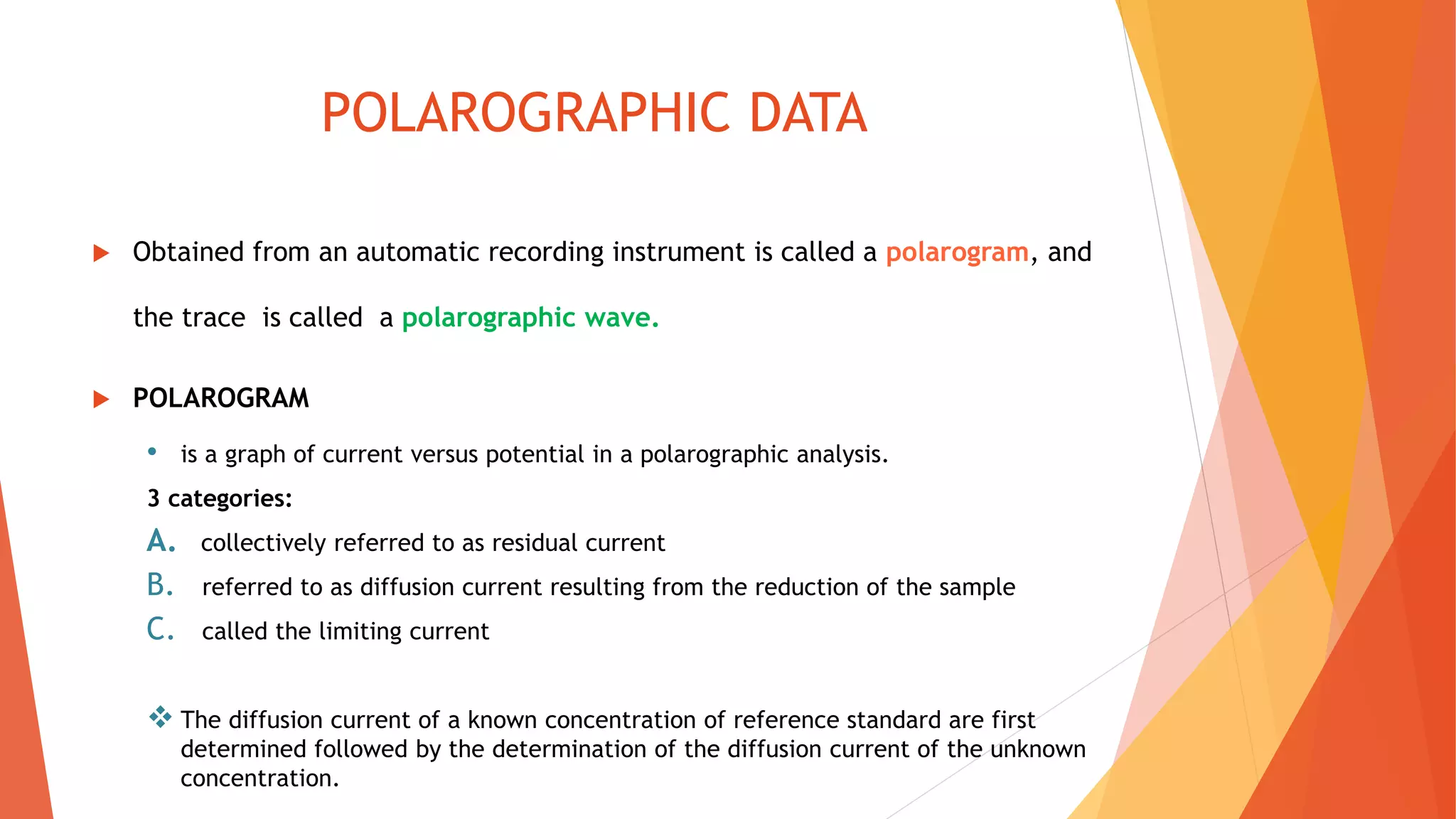
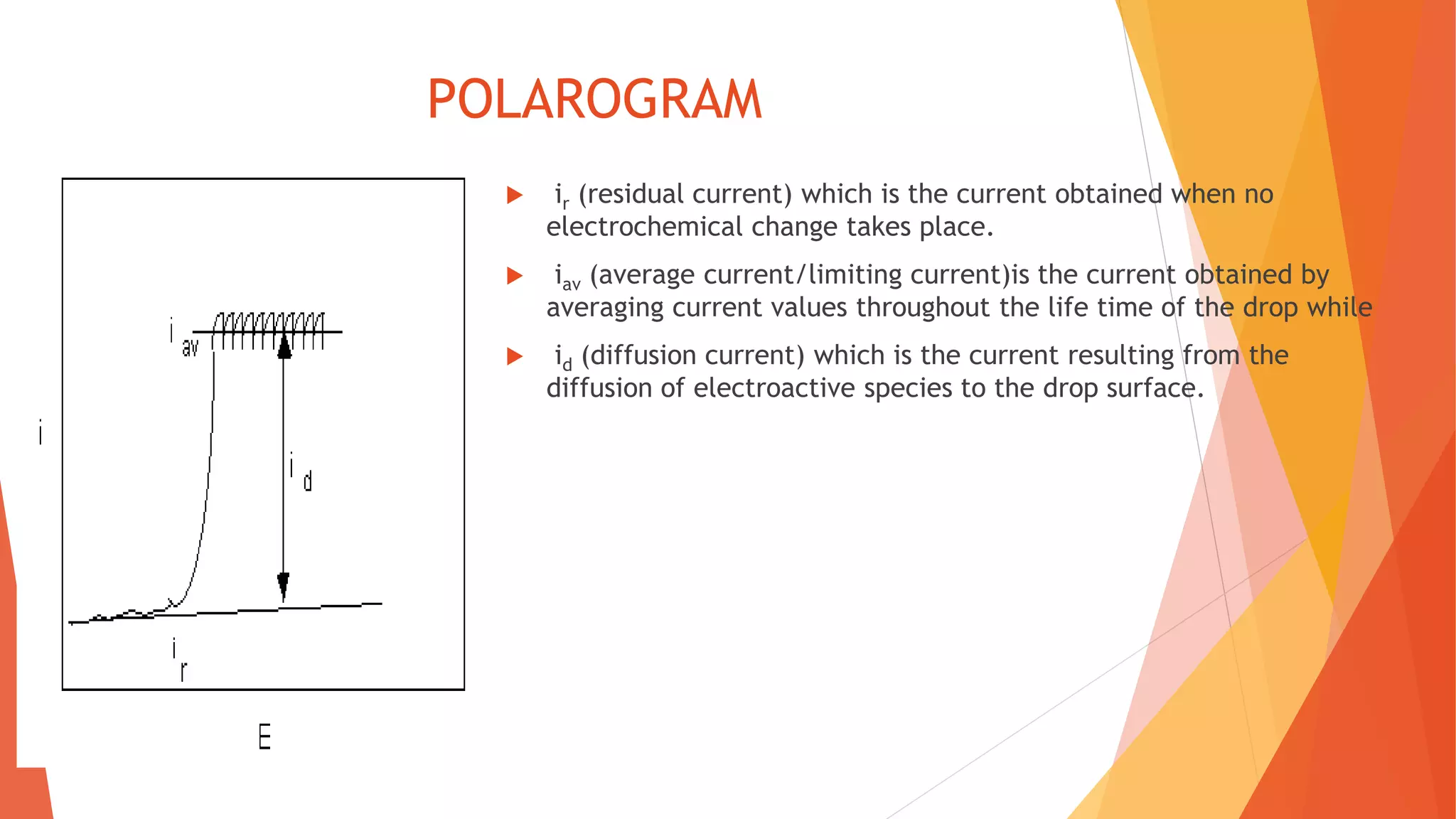
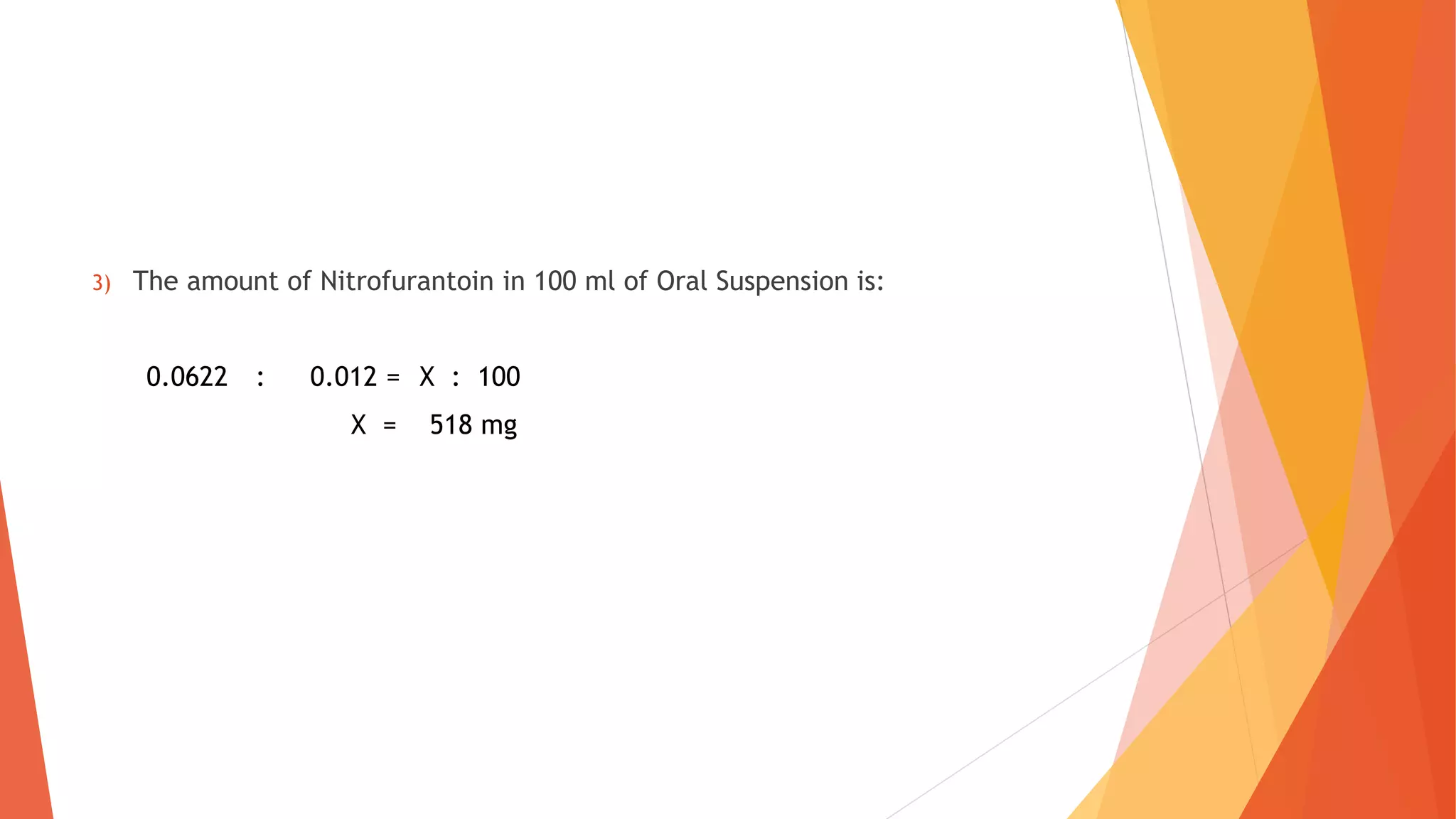
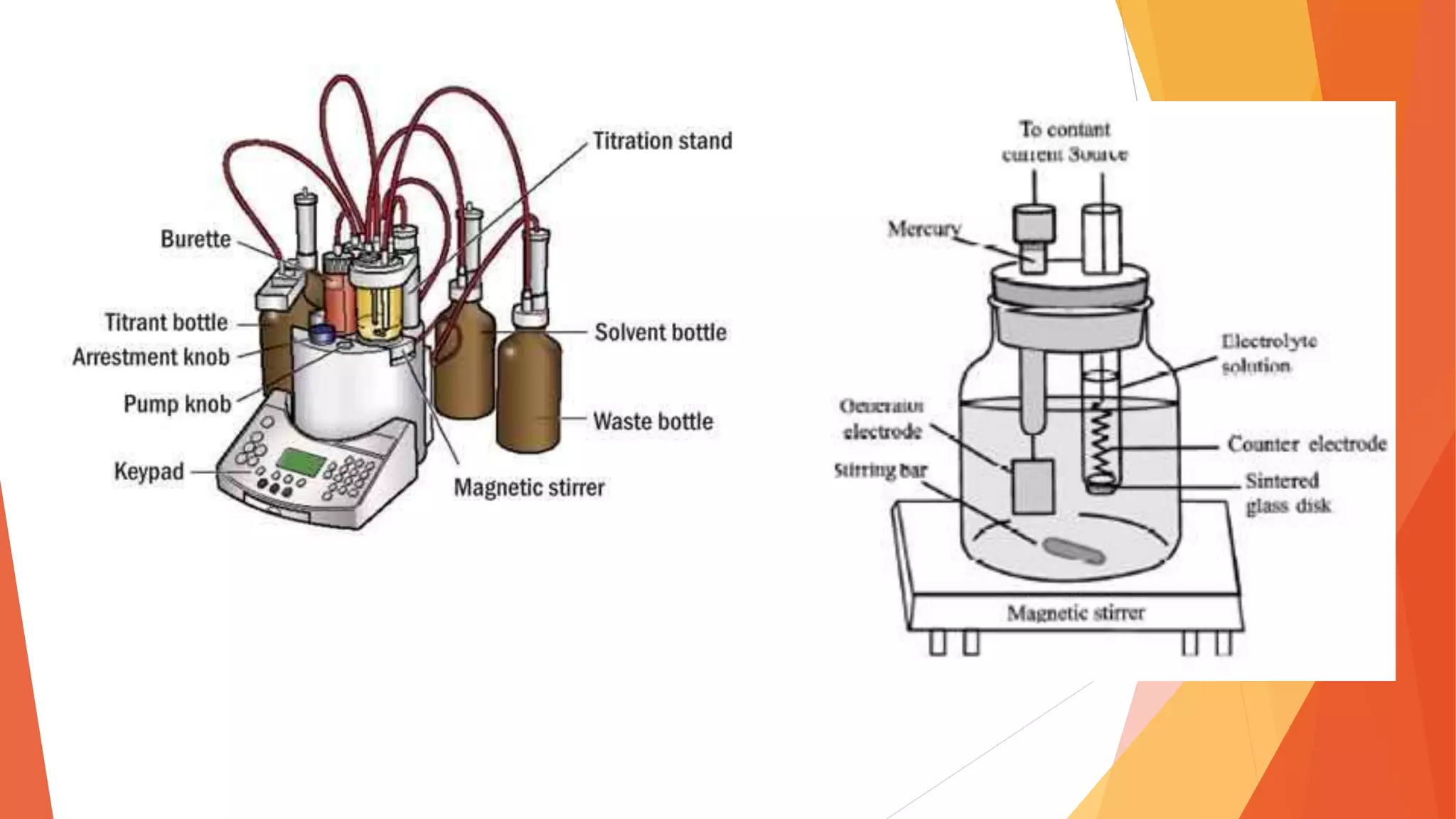
Polarography is an electroanalytical technique that uses a dropping mercury electrode to determine the concentration of electroactive species in a solution. It works by measuring the current flowing between the indicator electrode and reference electrode as the voltage is increased. Polarography provides a polarogram graph of current vs voltage that shows diffusion currents and limiting currents to identify species present. It has advantages like simple sample handling, speed, sensitivity and limited use of organic solvents. Polarography is used in pharmaceutical analysis to determine concentrations of drugs, vitamins, hormones and other compounds.