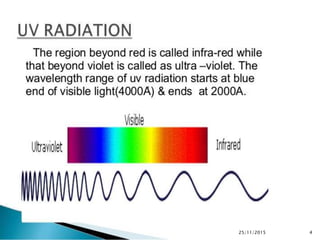
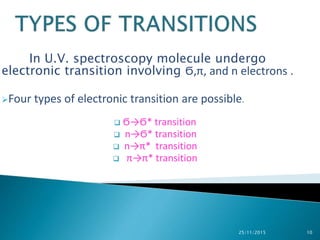
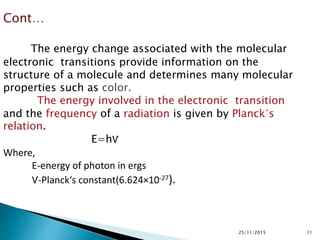
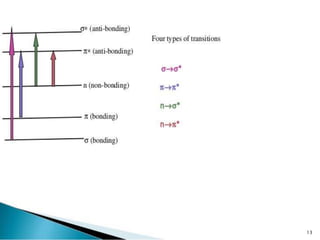
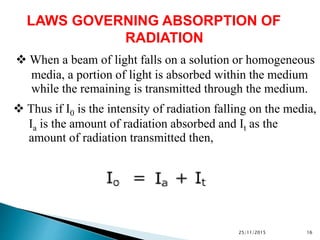
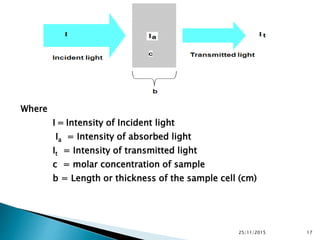
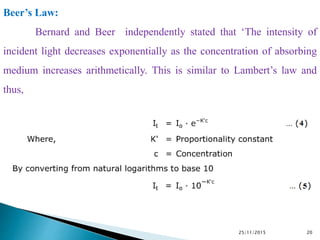
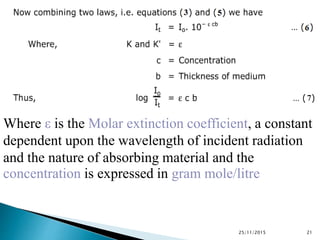
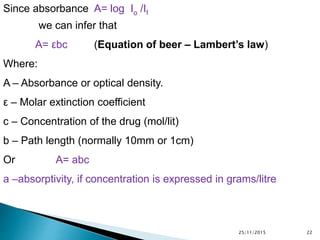
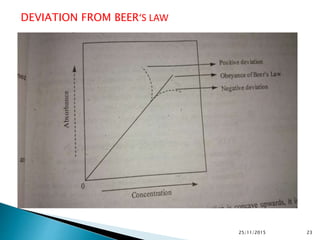
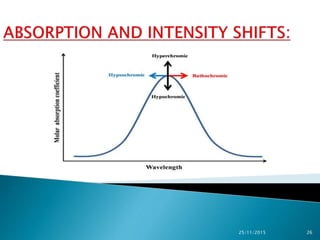
The document discusses ultraviolet-visible spectroscopy and its applications in analyzing molecular structure. It describes the four types of electronic transitions that can occur in molecules when exposed to UV-VIS light: sigma-to-sigma, n-to-sigma, n-to-pi, and pi-to-pi transitions. Beer's Law and Lambert's Law are introduced, relating absorbance to concentration, path length, and molar absorptivity. Deviations from Beer's Law can occur due to chemical changes in the sample or limitations of the instrumentation. Chromophores and auxochromes are defined as groups that impart or shift color to compounds.