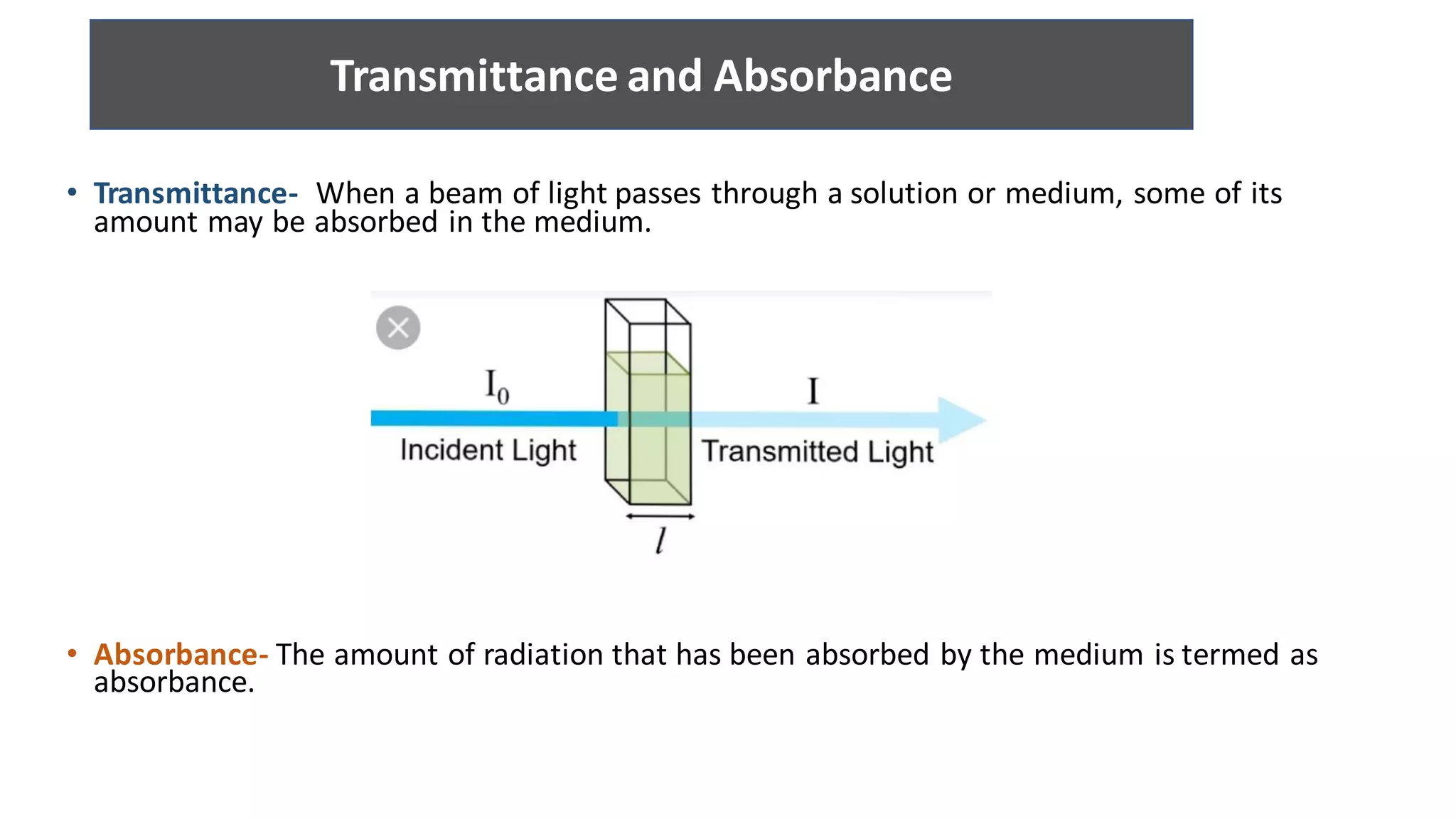
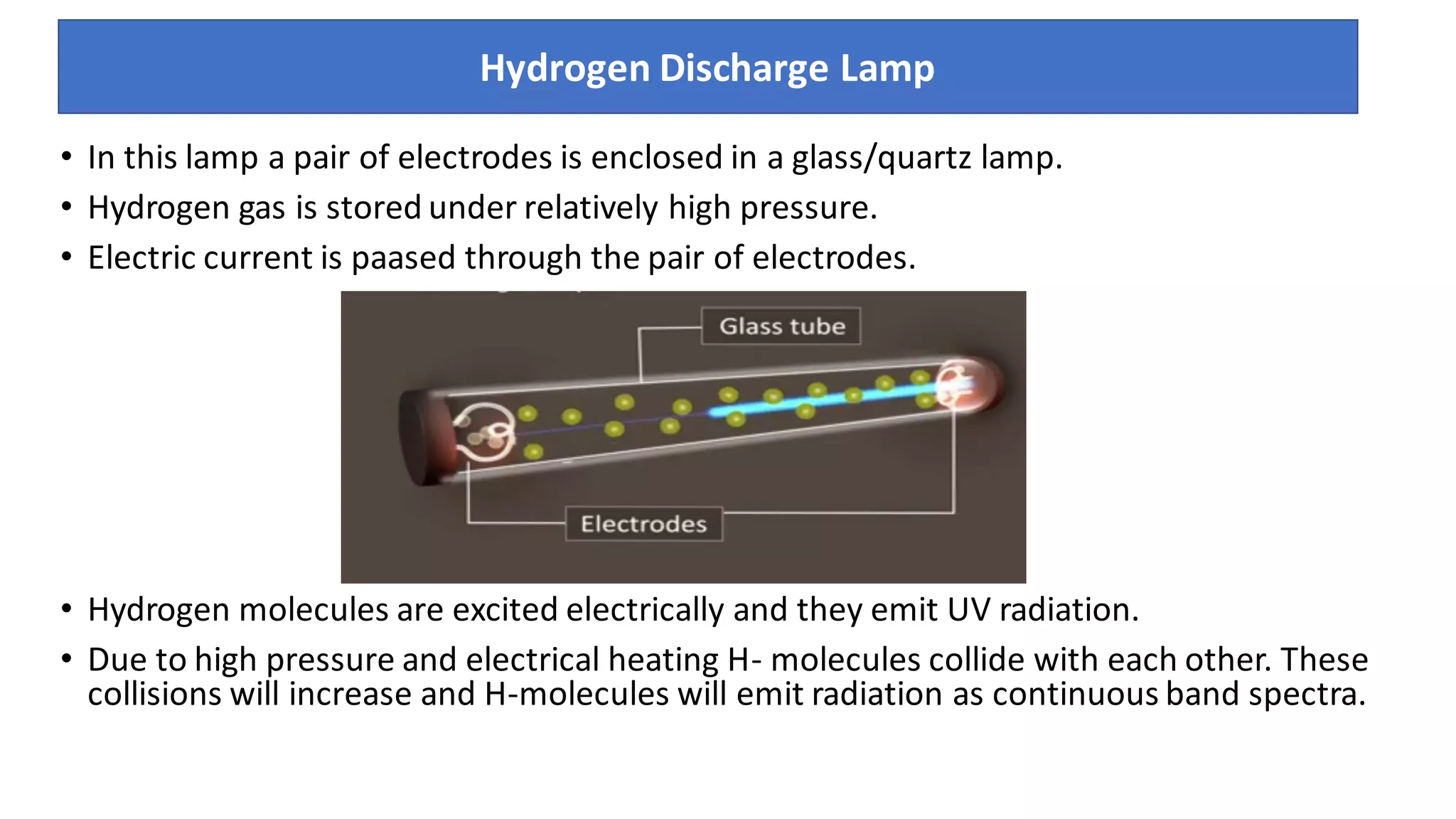
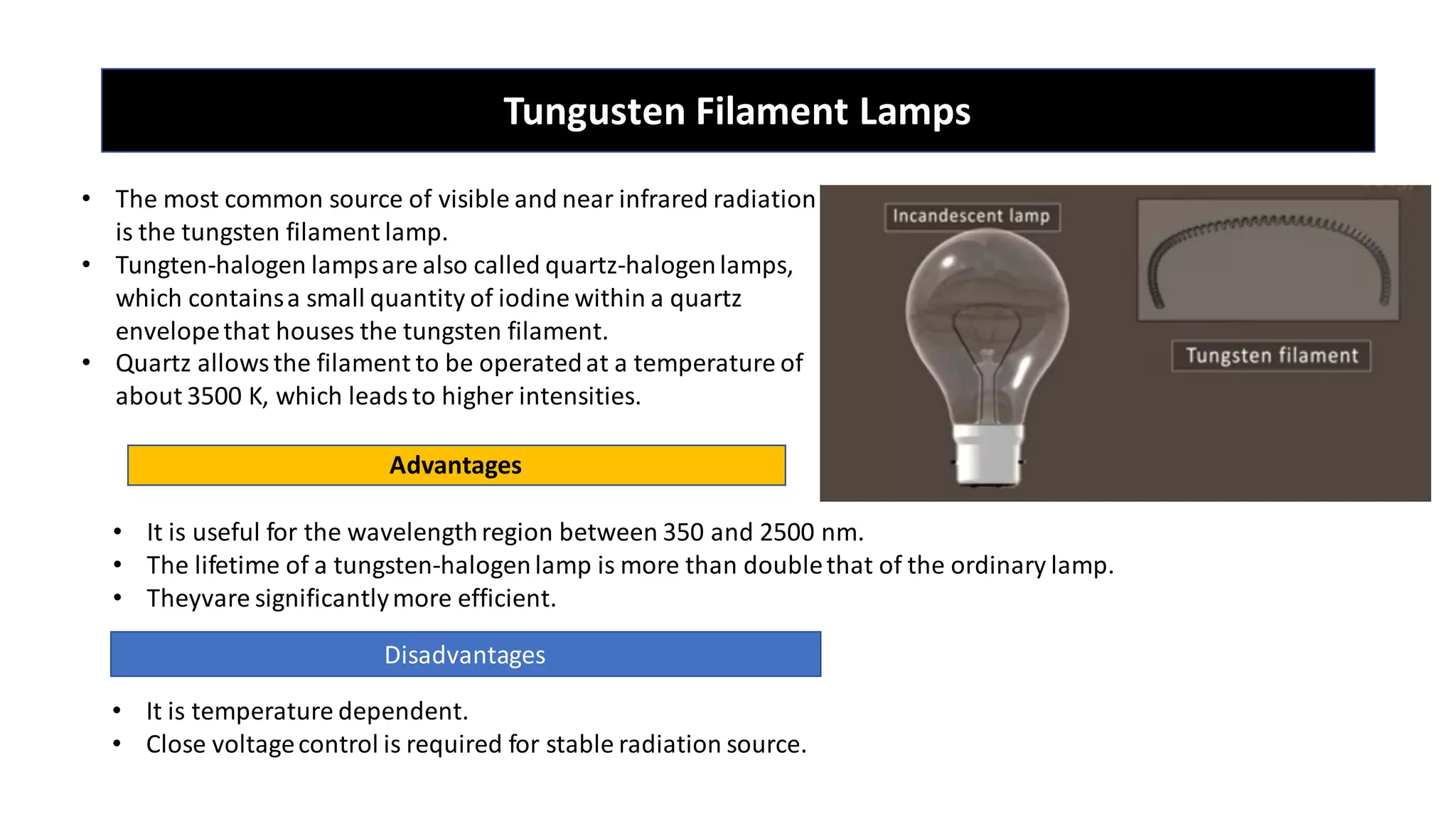
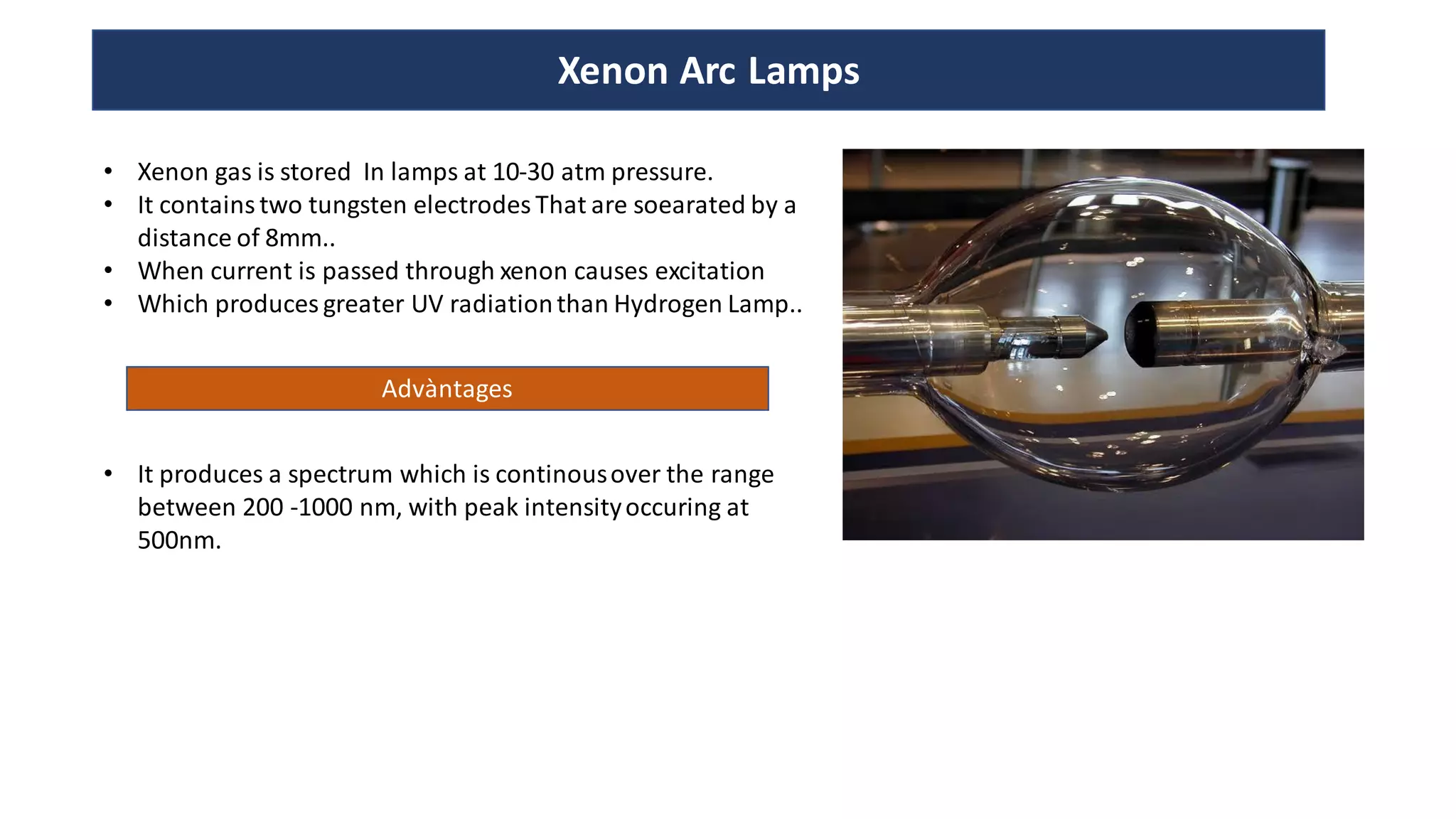
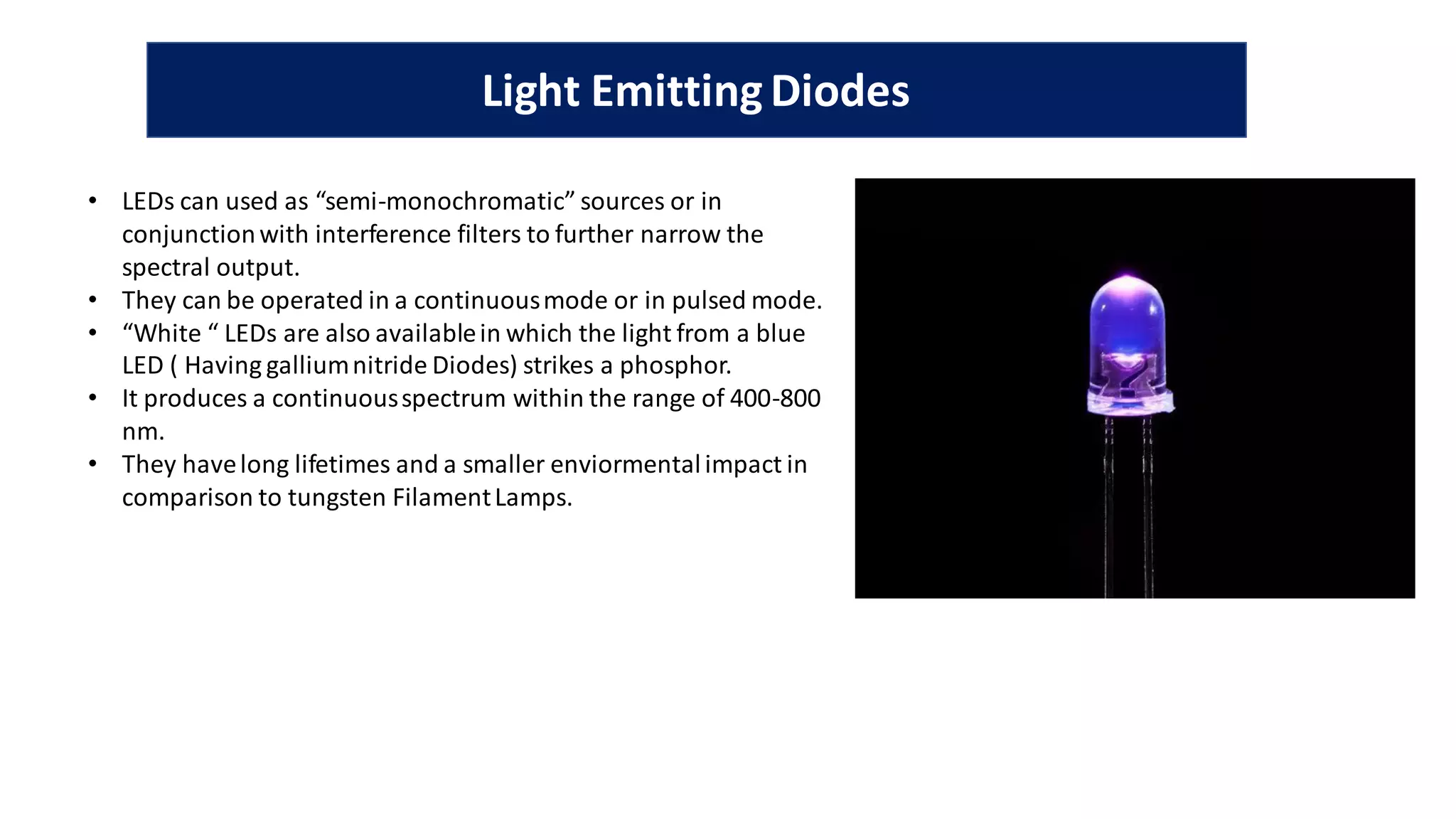
The document is a presentation on UV-Visible spectroscopy and Lambert's Beer Law, explaining concepts like transmittance, absorbance, and the importance of different light sources used in spectroscopy. It discusses the application of Beer’s law in various fields, limitations, and detailed descriptions of light sources such as hydrogen, deuterium, tungsten filament, xenon arc, and mercury arc lamps. The presentation concludes by emphasizing the relevance of spectrophotometry in chemical analysis and quality assurance.