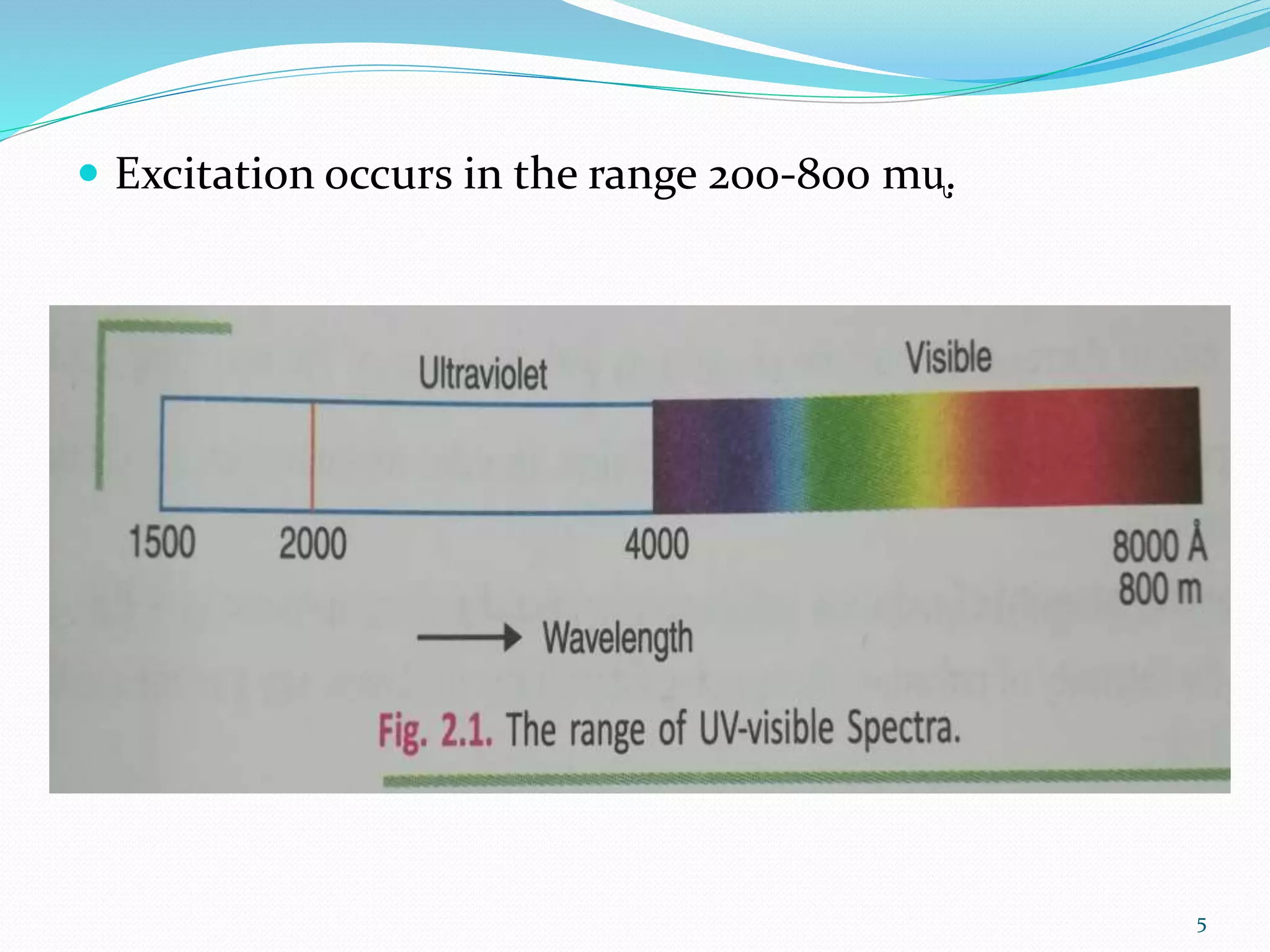
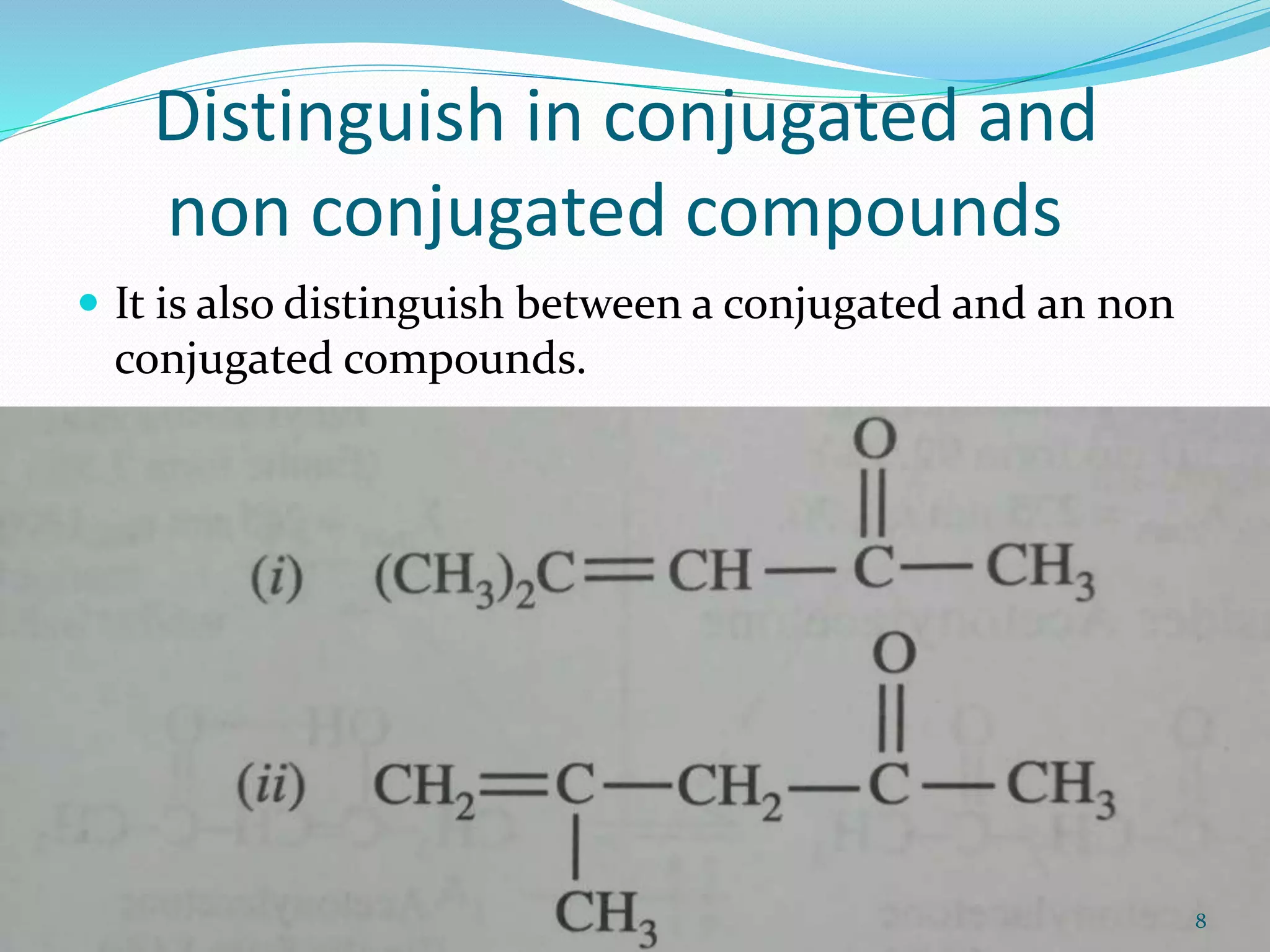
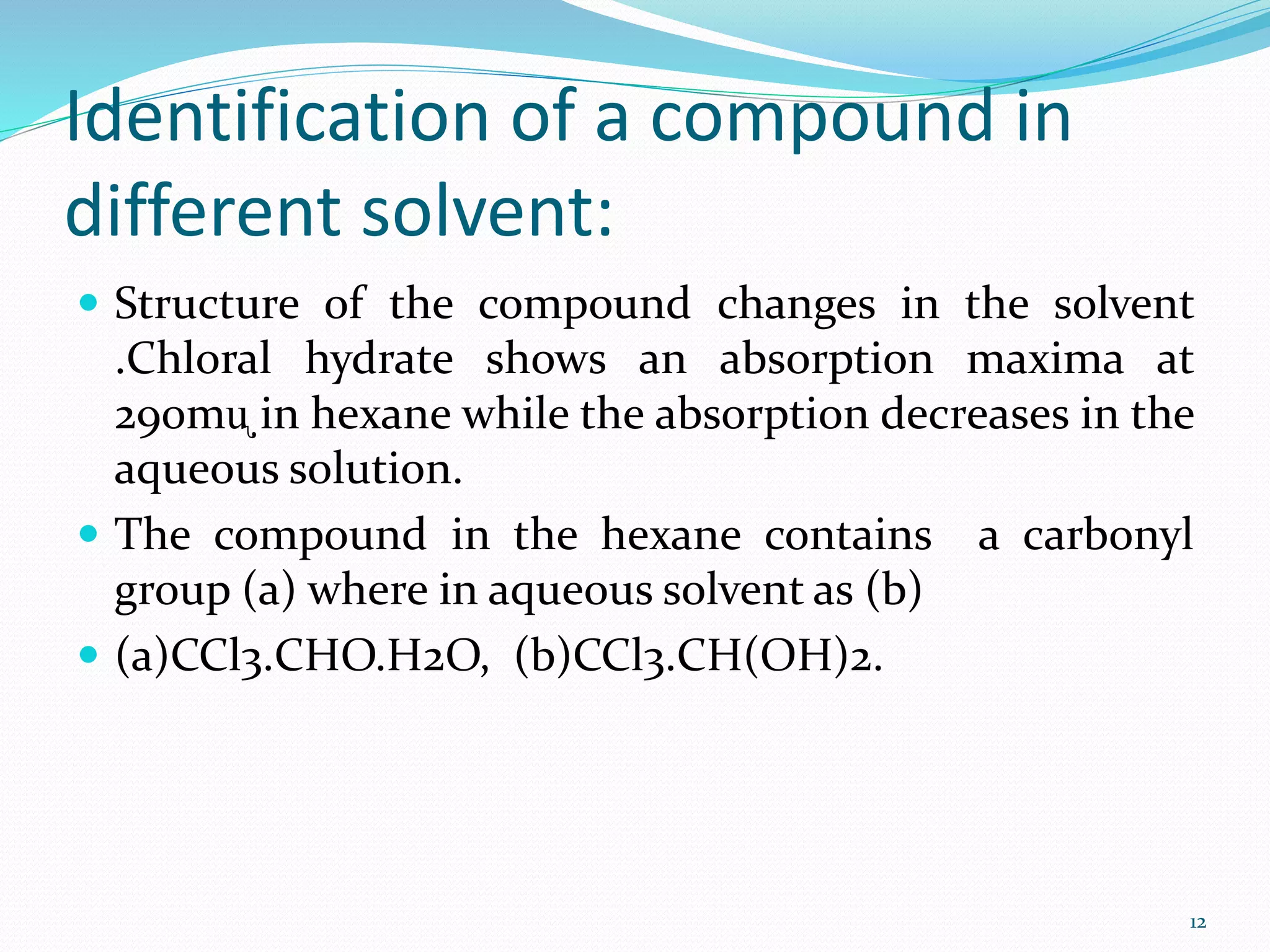
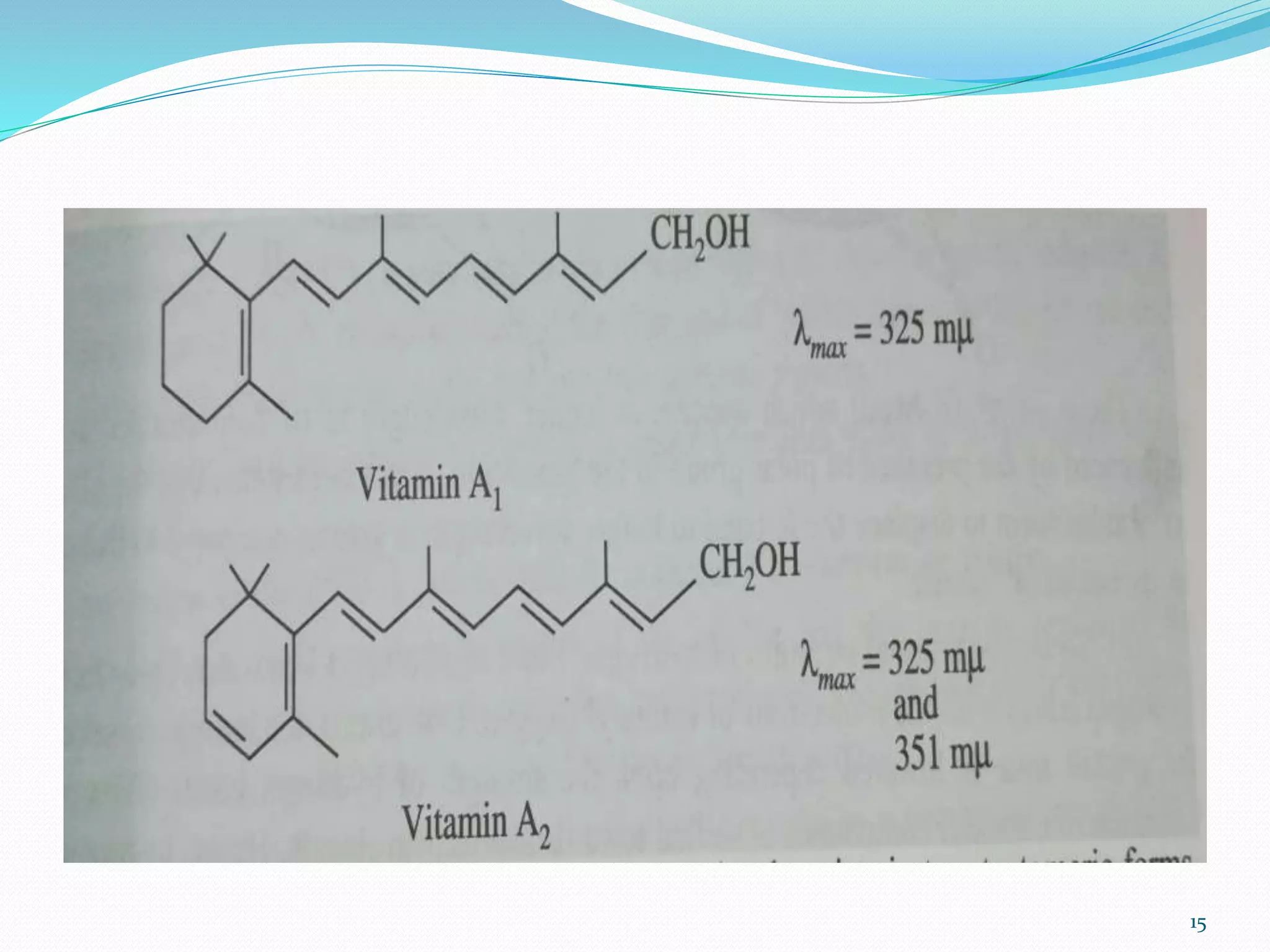
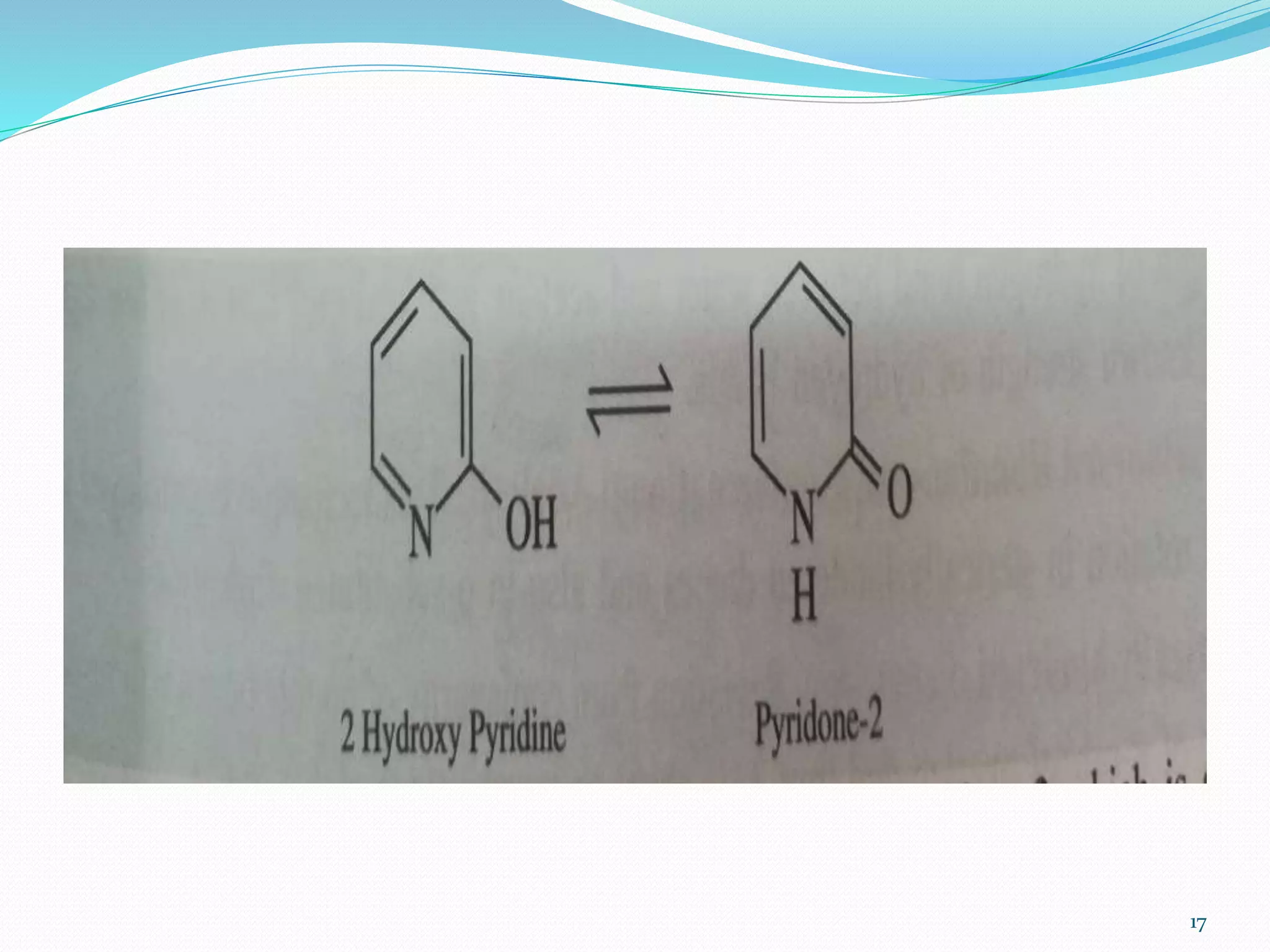
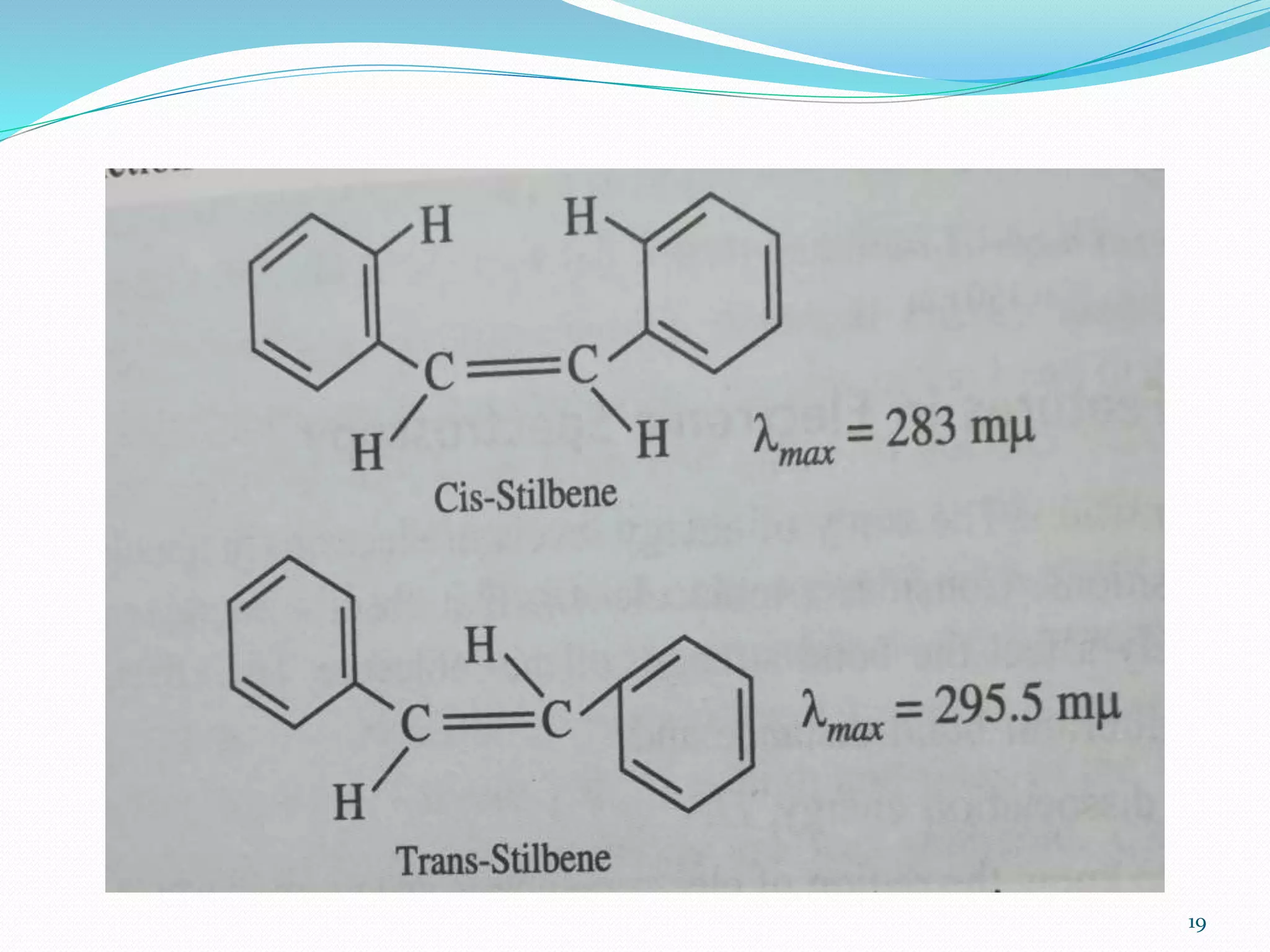
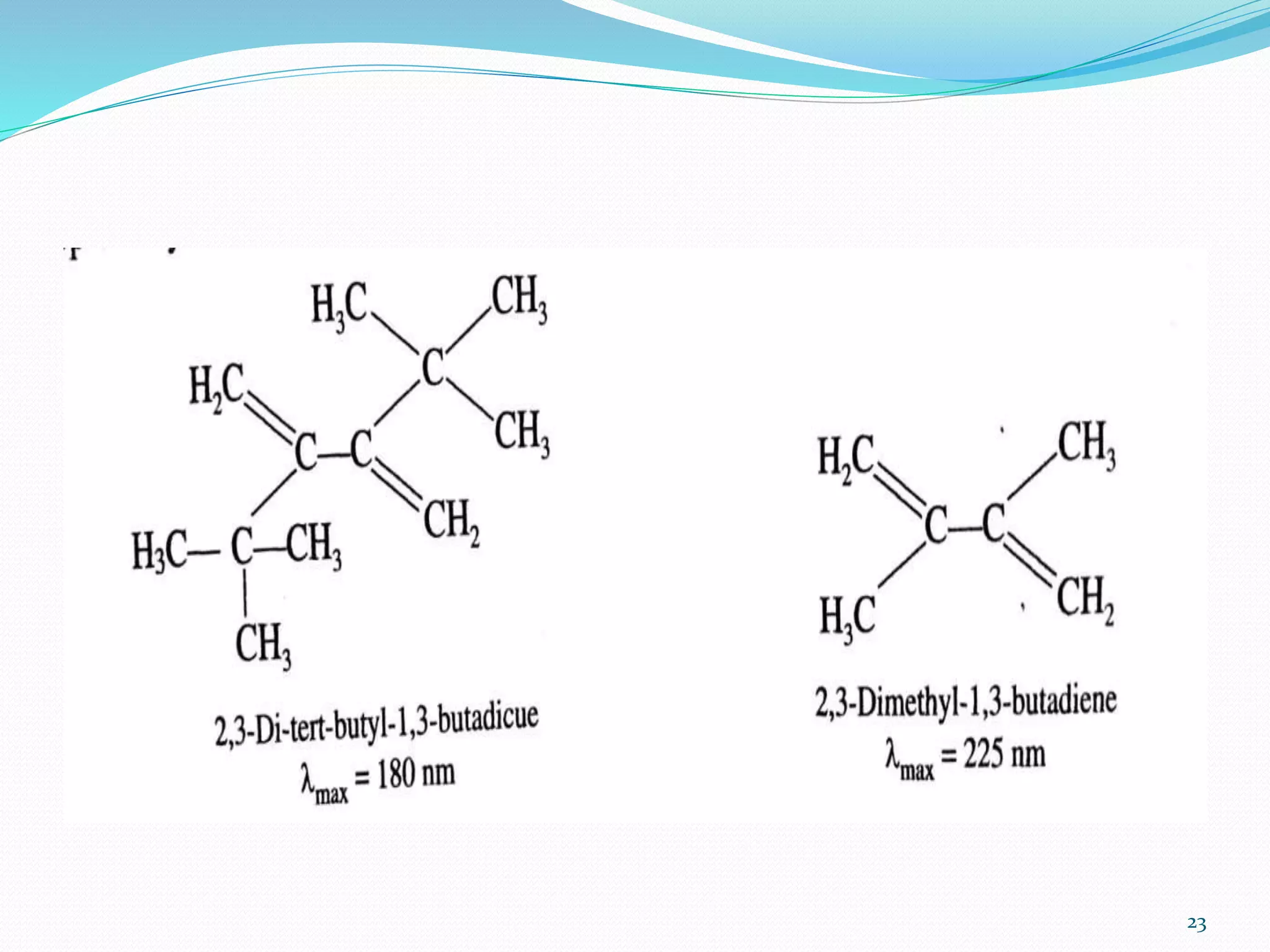
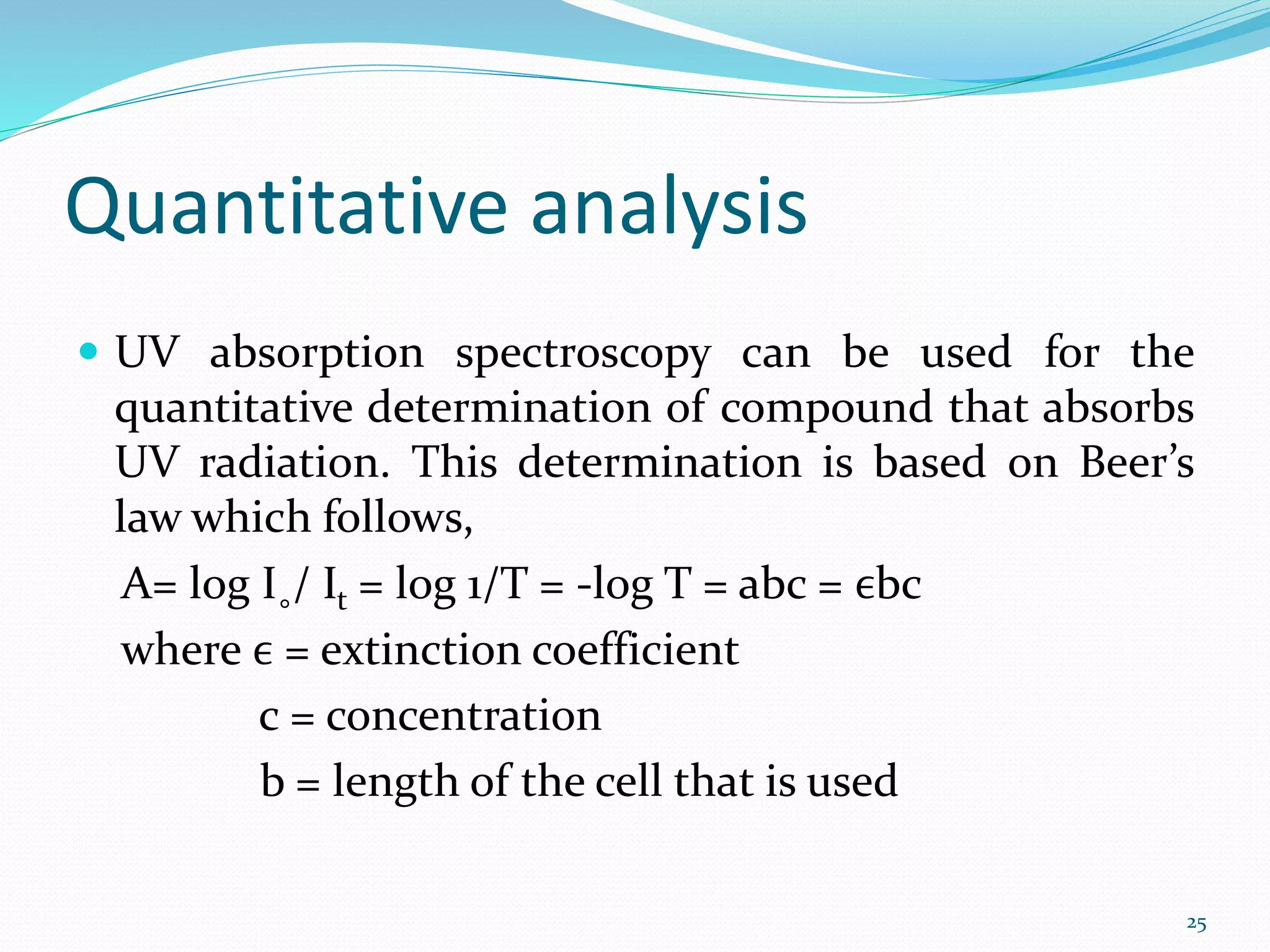
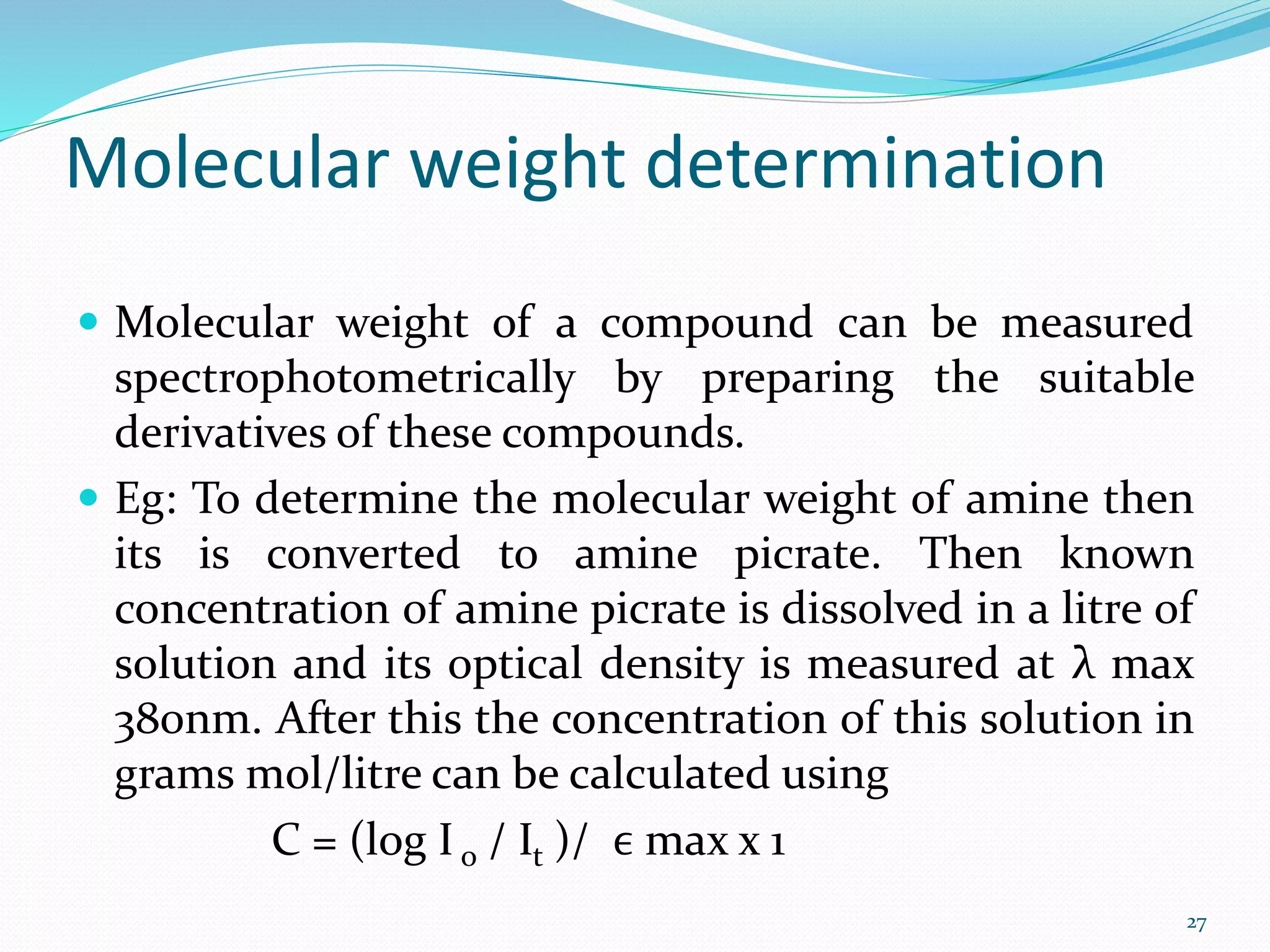
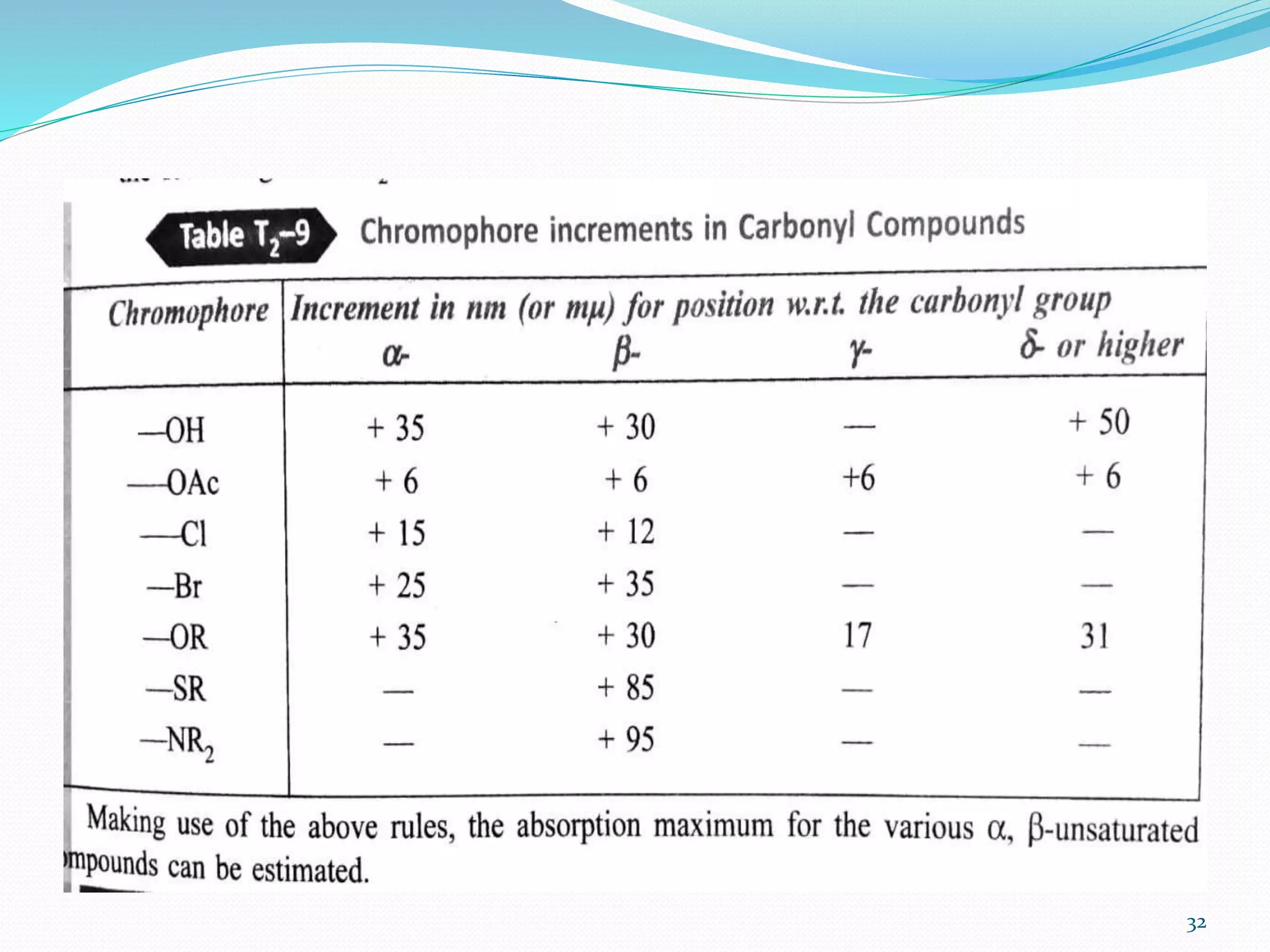
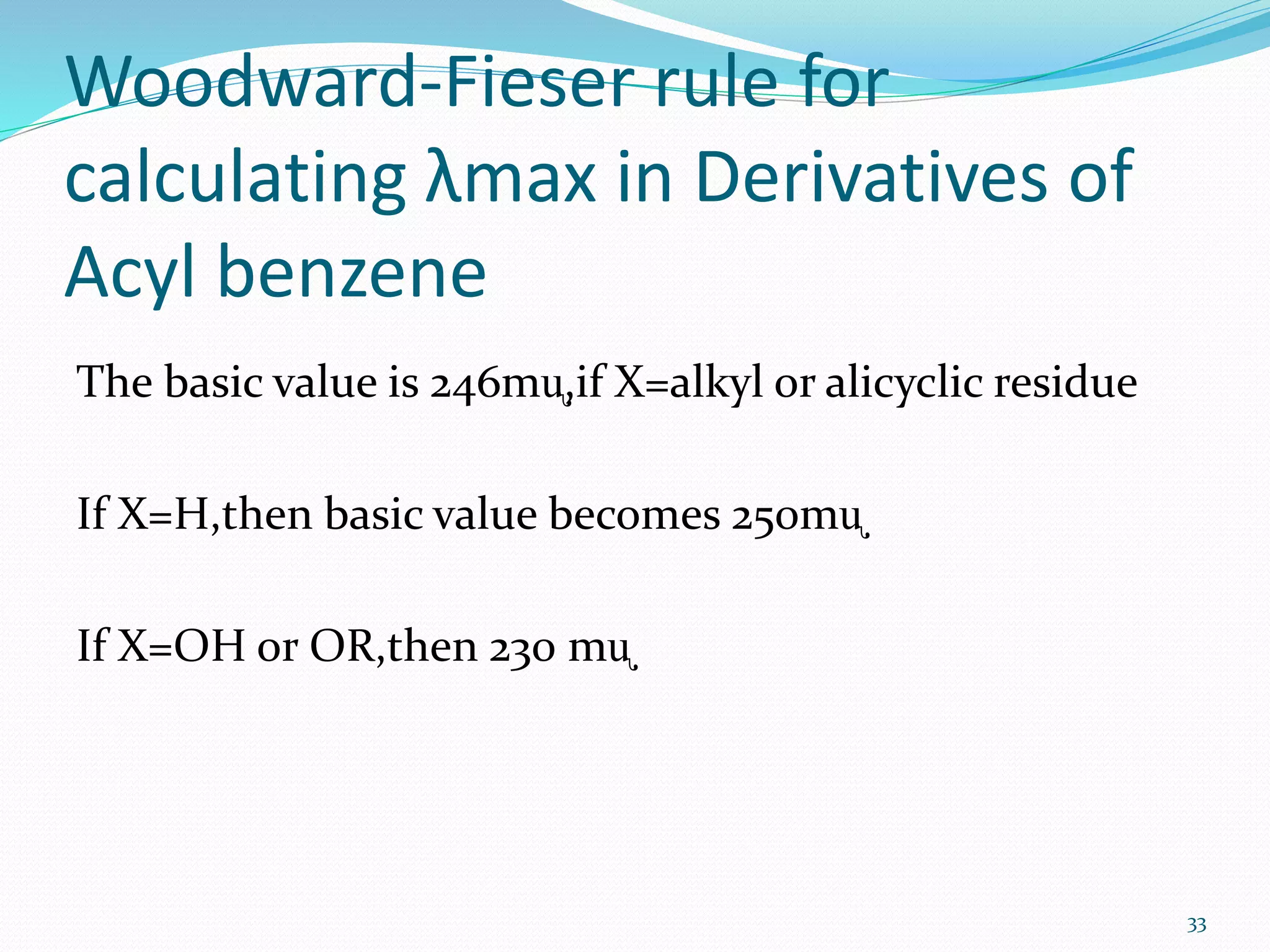
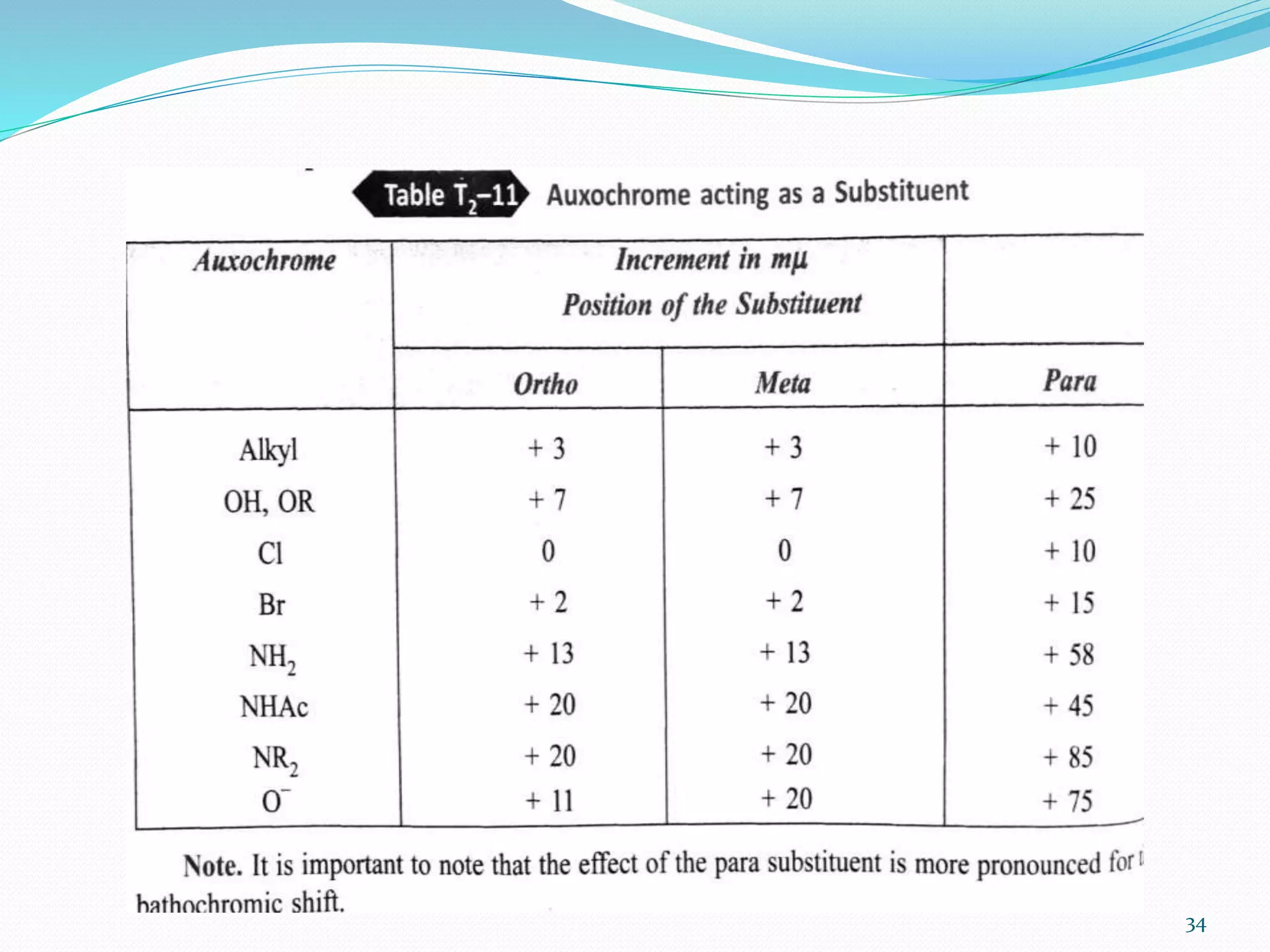
UV-visible spectroscopy involves measuring the absorption of light in the UV and visible light ranges. It is useful for determining conjugation and distinguishing between conjugated and non-conjugated compounds. It has applications in identifying unknown compounds, determining the extent of conjugation, and elucidating the structures of molecules like vitamins. It can also provide information about configuration, hydrogen bonding, molecular weight, and detect impurities. The Woodward-Fieser rules allow calculating the expected absorption maxima for certain functional groups.