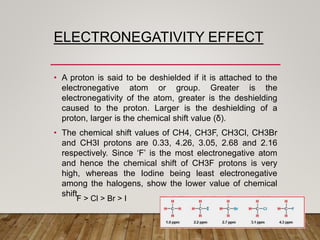
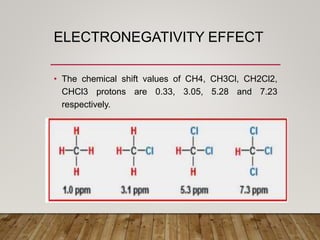
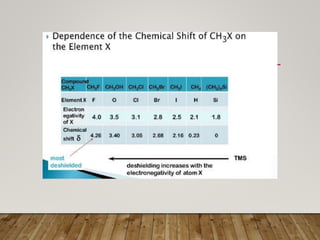
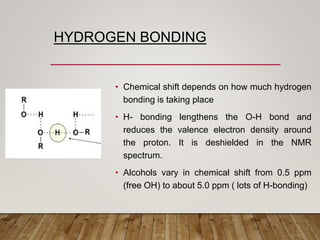
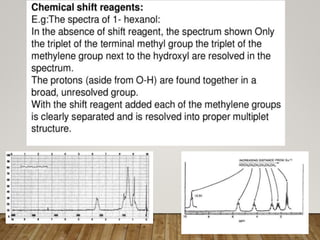
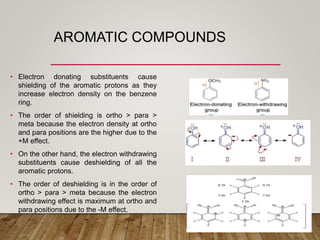
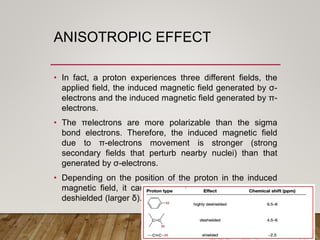
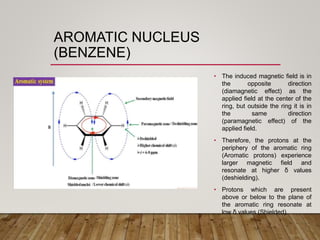
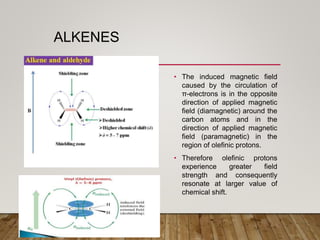
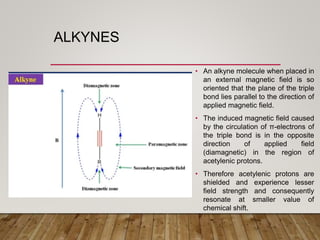
1) Chemical shift is defined as the shifts in NMR signals due to shielding or deshielding of protons by electrons in different chemical environments. (2) Factors that affect chemical shift include electronegativity, solvent effects, hydrogen bonding, and anisotropic effects. (3) Lanthanide shift reagents interact with functional groups, simplifying NMR spectra through induced chemical shifts.