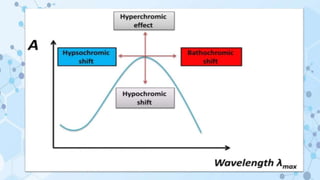
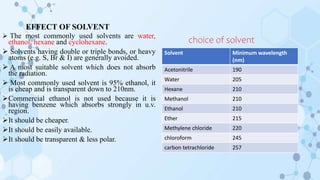
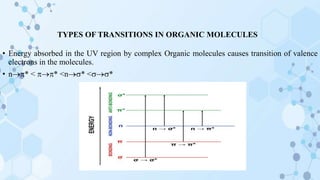
This document provides an overview of the basic theory of UV-visible spectroscopy. It discusses the origins of UV absorption and emission spectra from electronic transitions in molecules. The types of electronic transitions that can occur, such as n→π*, π→π*, and σ→σ* transitions, are explained. The effects of chromophores, auxochromes, and solvents are also summarized. Key concepts covered include electronic excitation and absorption, molecular orbitals, bonding and antibonding orbitals, and the factors that influence spectral peak positions.


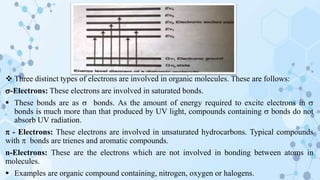




![CHROMOPHORE
Chromophore is defined as any group which exhibits absorption of electromagnetic
radiations in the visible or ultraviolet region.
Important chromophore are ethylenic, carbonyls, ester acid, nitrile group etc
Two types of chromophore are known;
a) chromophore in which the group is having electrons undergo * transition. Example of
having such chromophore are ethylene, ester. [Also called Dependent chromophore When more
then one chromophore is required to produce color. Eg acetone having 1 kentone group is
colorless where as diacetyl having two kentone group is yellow ]
b) Chromophore, having both electron and {non-bonding} electrons undergo two type of
transition, i.e * and n *. Example carbonyls, nitrile, azo compound, nitro
compound.[Also called Independent chromophore: single chromophore is sufficient to import
color to the compound ]](https://image.slidesharecdn.com/basictheoryofuvvisiblespectroscopy-231224073553-5c1960e1/85/BASIC-THEORY-OF-UV-VISIBLE-SPECTROSCOPY-pptx-11-320.jpg)