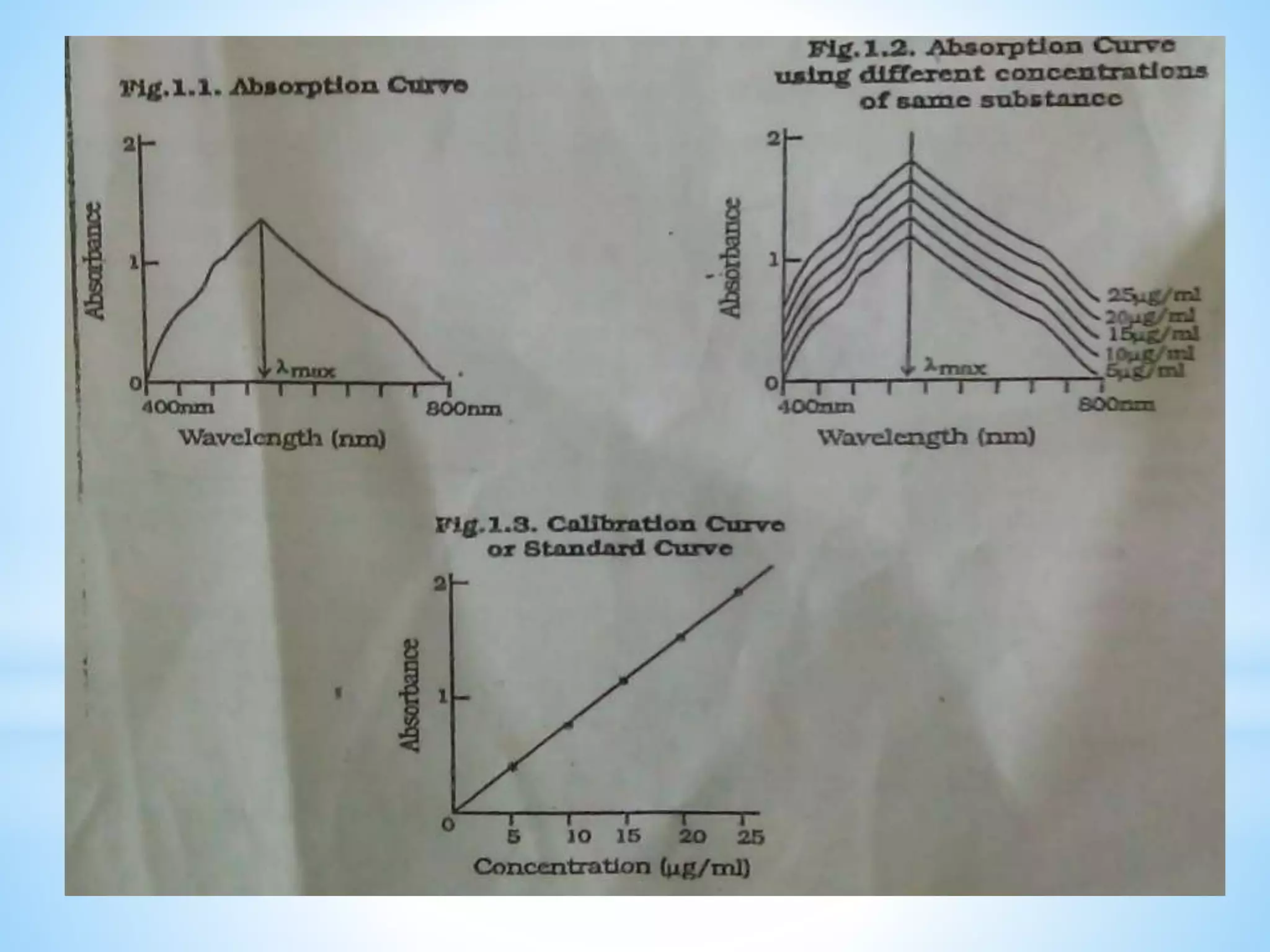
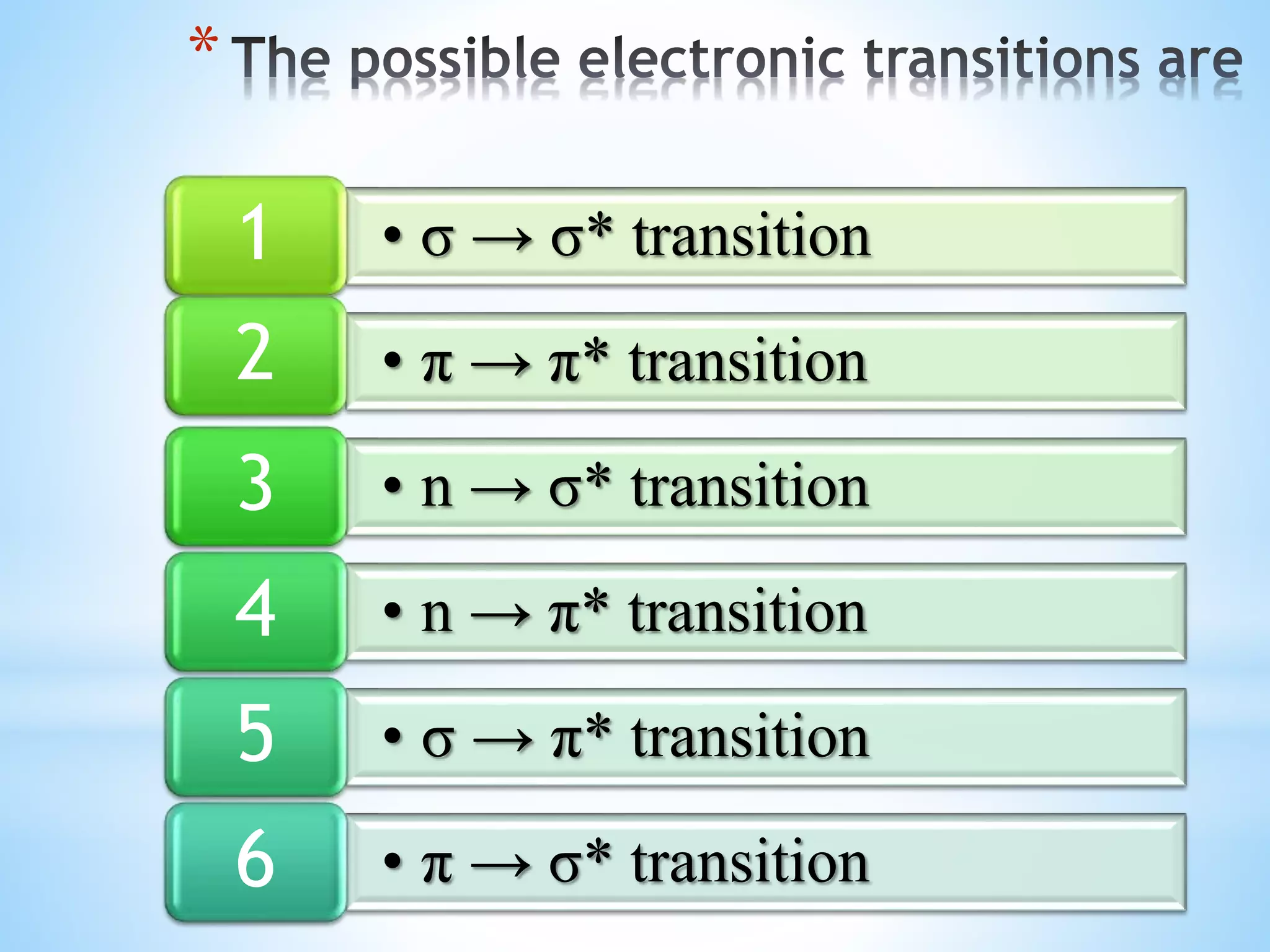
The document discusses UV-visible spectroscopy, which involves measuring the absorption of ultraviolet or visible radiation by molecules as they transition between energy levels. It explains the basic concepts of spectroscopy including electromagnetic radiation, absorption curves, electronic transitions, and Beer's and Lambert's laws which describe the relationship between absorbance and analyte concentration. The principles of UV-visible spectroscopy are useful for qualitative and quantitative analysis of compounds in various applications.