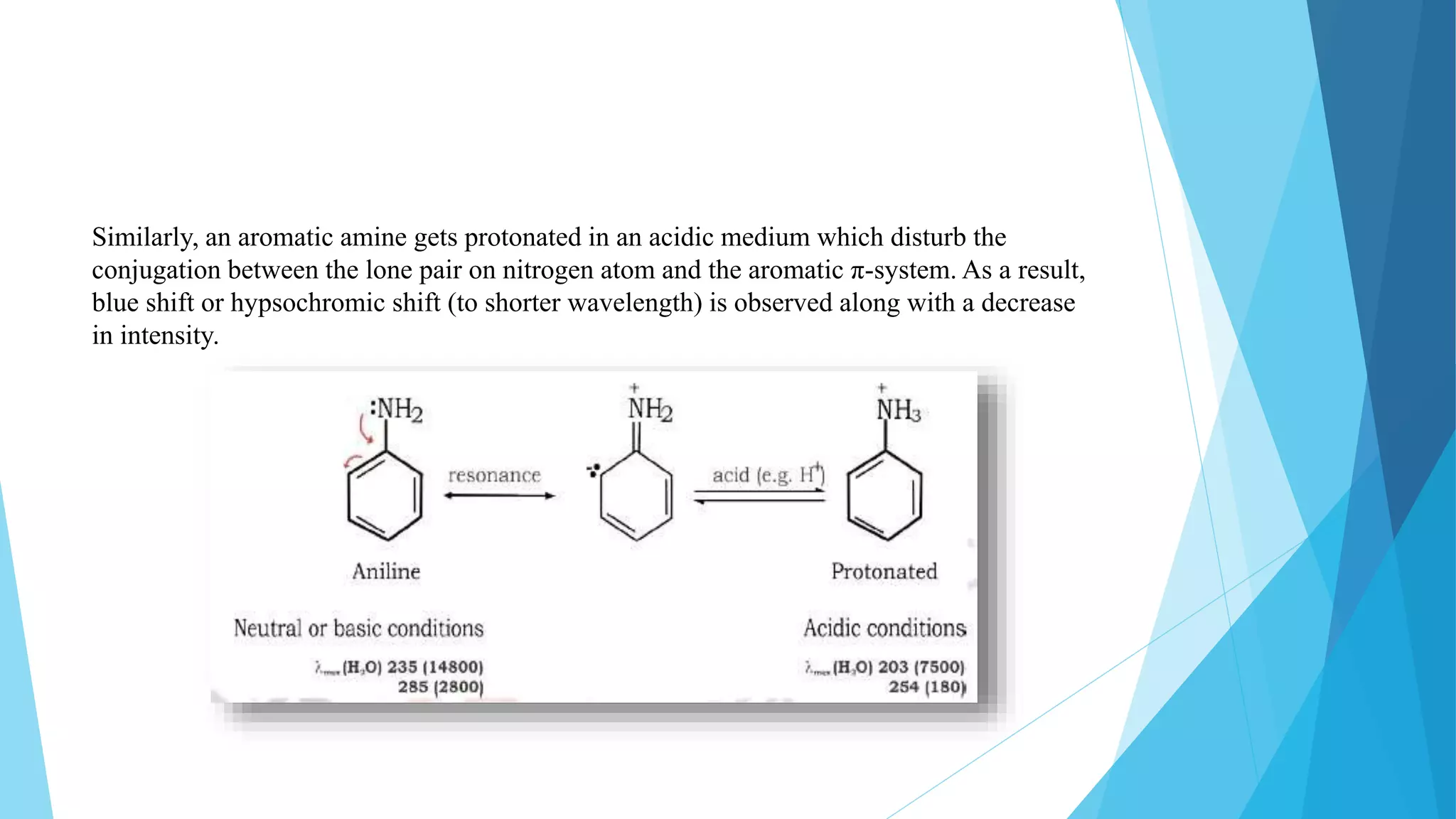
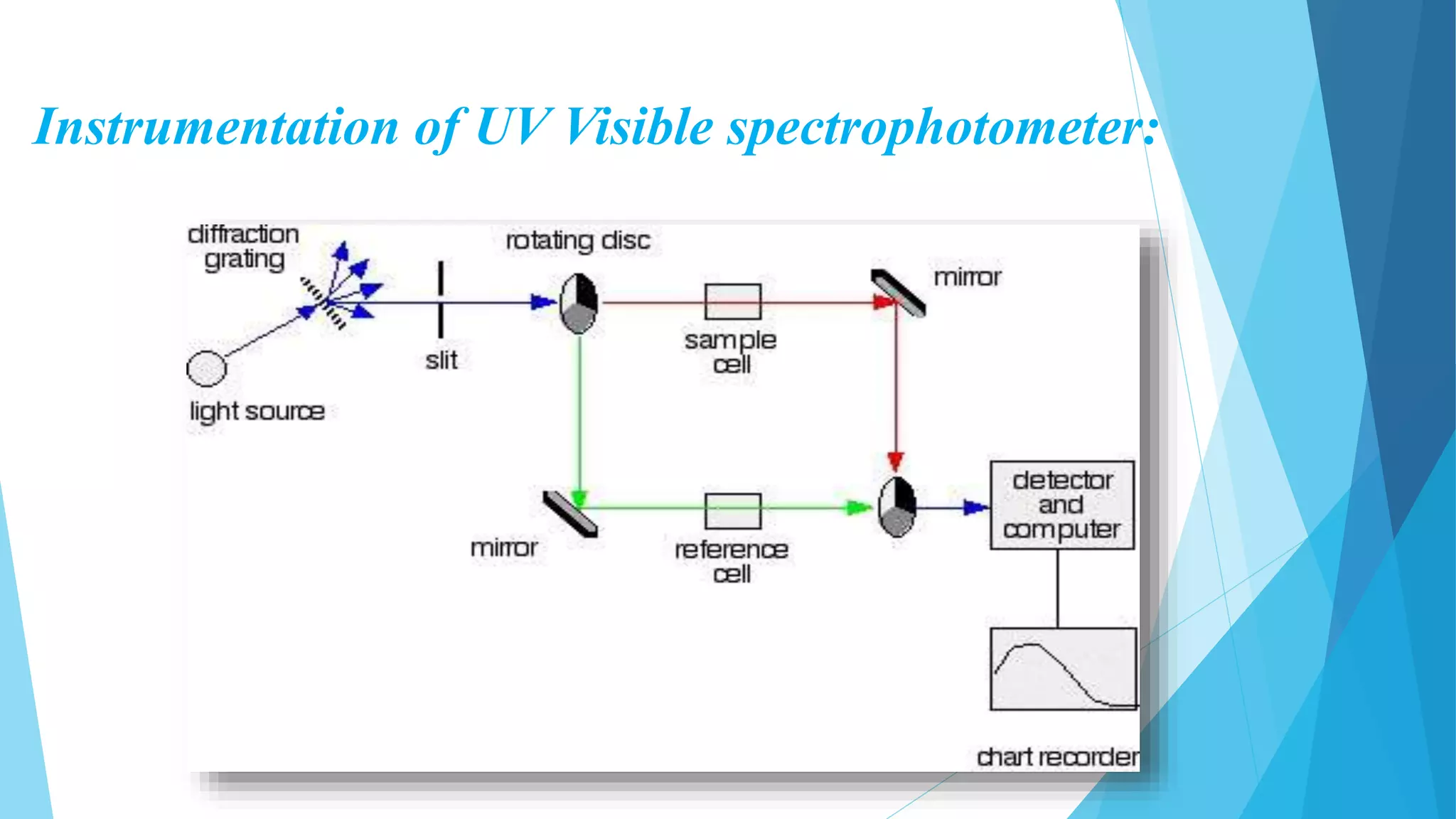
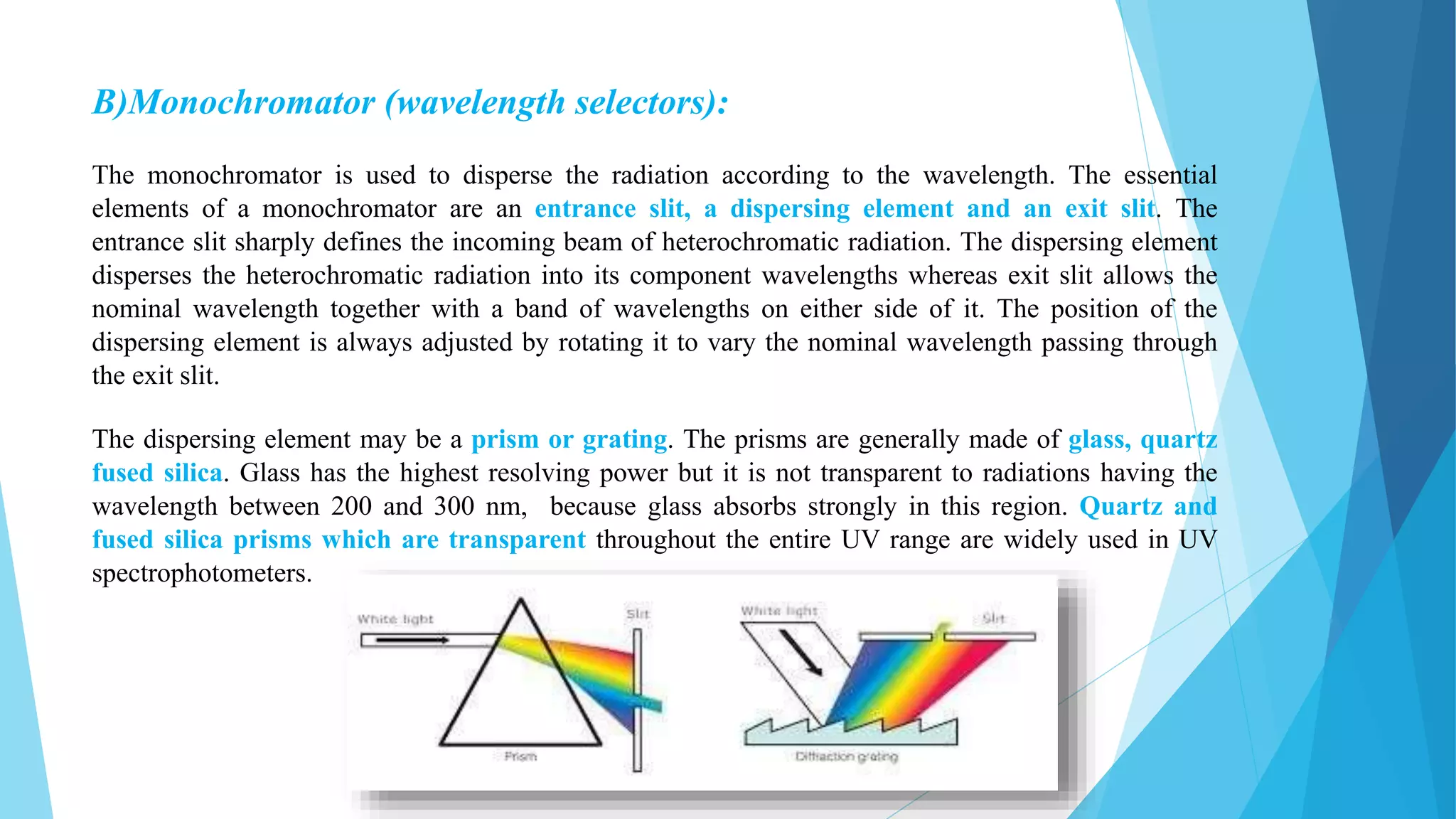
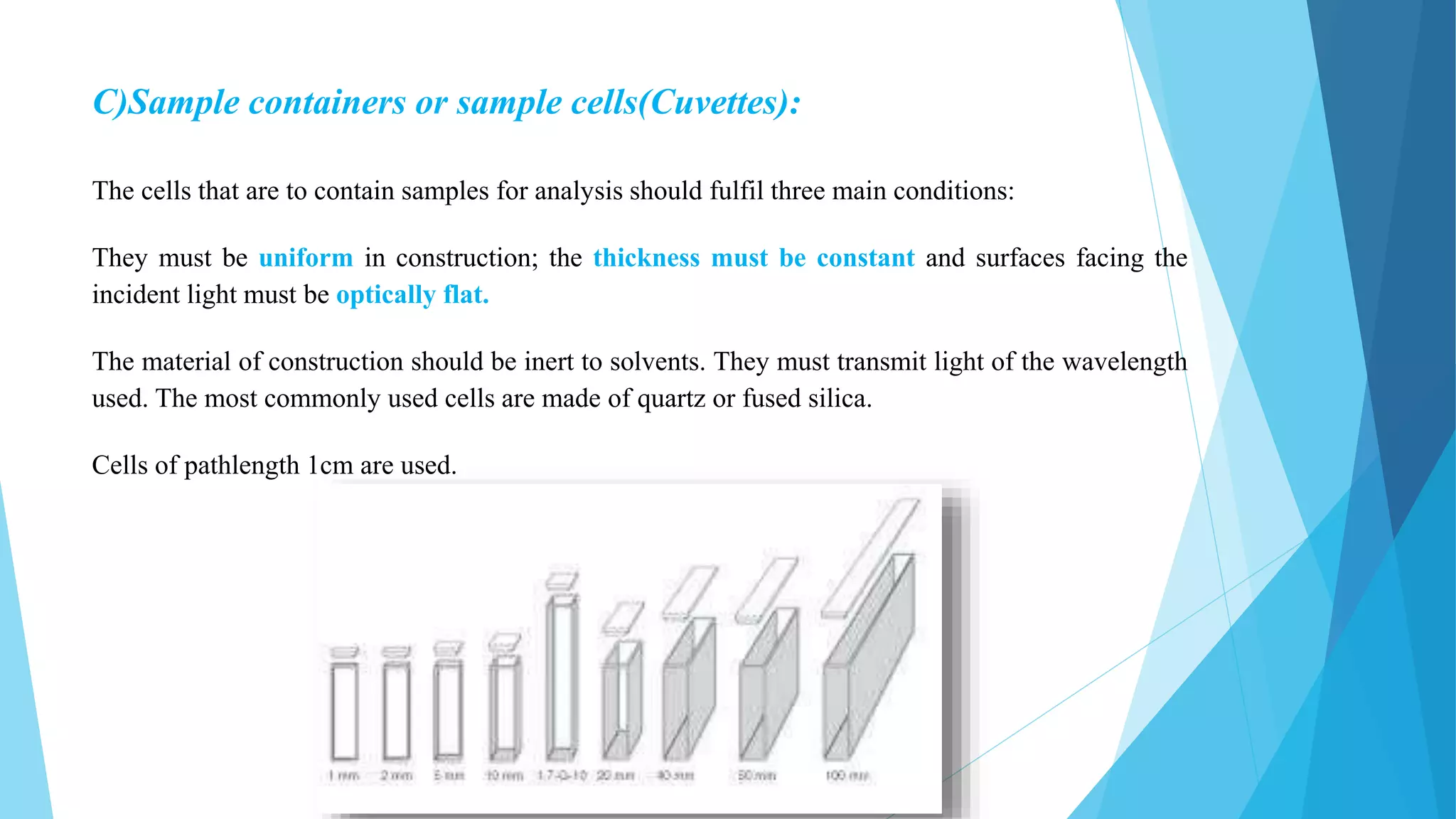
UV-Visible Spectroscopy is a technique that uses light in the visible and adjacent ranges. It works based on how molecules absorb light at specific wavelengths. The document discusses the principles of UV-Vis spectroscopy including Beer's law, electronic transitions, chromophores and auxochromes. It also covers instrumentation components like light sources, monochromators, sample cells and detectors. Factors affecting absorption spectra are solvents, pH and conjugation. The technique has applications in analytical chemistry for identification and quantification of analytes.