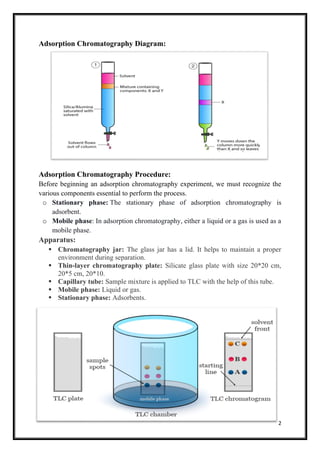
Adsorption chromatography is a technique for separating components in a mixture based on differential adsorption of the components onto a stationary solid phase. It works by passing a mobile liquid or gas phase over an adsorbent stationary phase in a column, which causes components to separate as they are differentially retained on the surface of the adsorbent. Common types include thin layer chromatography, paper chromatography, and column chromatography. Adsorption chromatography has various applications such as separating amino acids, isolating antibiotics, and identifying carbohydrates.