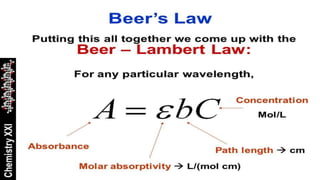
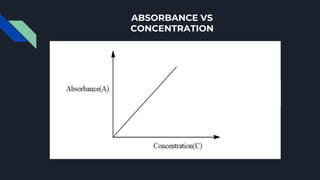
The Beer-Lambert law describes the relationship between the concentration of a substance in solution and the amount of light it absorbs. It states that absorbance is directly proportional to concentration and path length. Mathematically, it can be expressed as: A = ε * c * l, where A is absorbance, ε is the molar absorptivity, c is concentration, and l is path length. The Beer-Lambert law is important in quantitative analysis using UV-visible spectroscopy. It is only applicable to monochromatic light and at low concentrations where molecule interactions can be ignored.