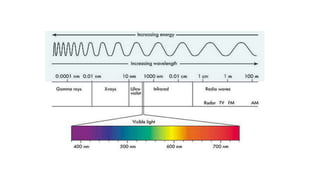
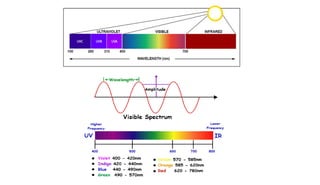
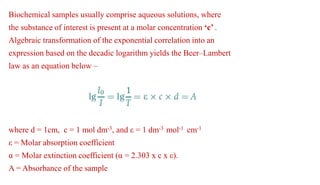
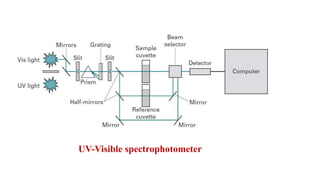
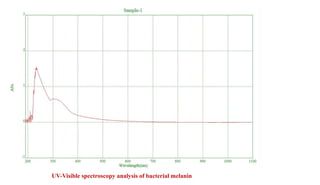
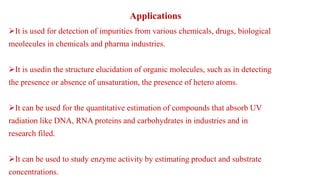
Ultraviolet and visible light spectroscopy (UV-Vis spectroscopy) measures the absorption and transmission of light by chemical compounds and biological molecules. It works by exciting electrons in molecules to higher energy states when different wavelengths of light pass through a sample. The intensity of light is measured before and after passing through the sample. The Beer-Lambert law states that absorbance is directly proportional to concentration, path length, and extinction coefficient. A spectrophotometer contains a light source, monochromator, sample and reference cells, detector, and recording devices to measure absorbance across wavelengths and determine concentrations of unknown samples. Common applications of UV-Vis spectroscopy include identification of organic compounds and quantification of biomolecules.