Embed presentation
Downloaded 158 times
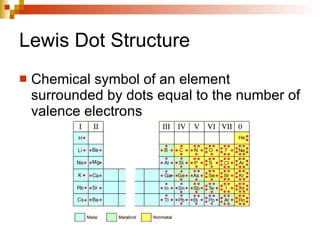
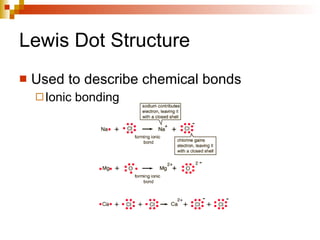
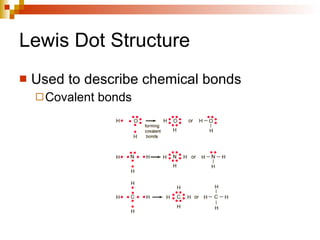
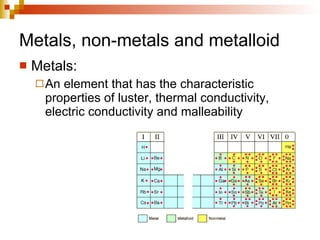

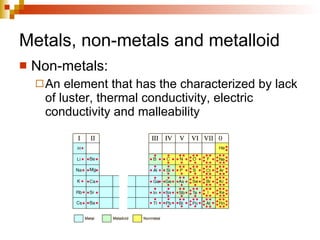
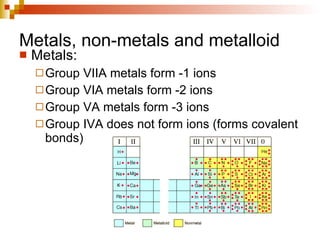
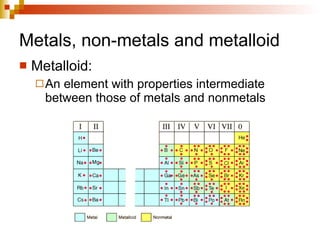
Lewis dot structures represent elements with dots around their symbols equal to their number of valence electrons and are used to describe ionic and covalent chemical bonds. Metals are elements that are lustrous, conductive of heat and electricity, and malleable. They include groups IA, IIA, and IIIA, which form 1+, 2+, and 3+ ions respectively. Non-metals lack luster, conductivity, and malleability, while metalloids have properties between metals and non-metals.








