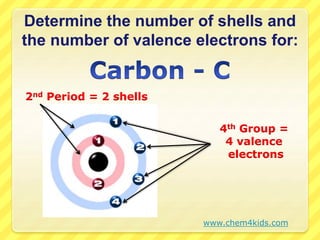
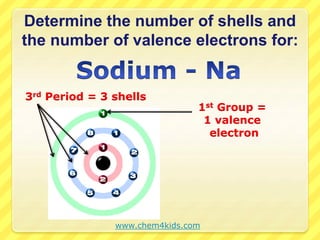
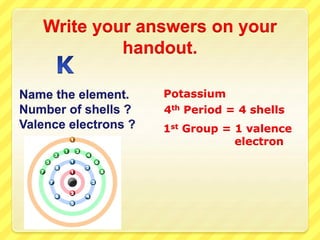
The document discusses the periodic table and properties of elements. It explains that each row of the periodic table is called a period, with elements in the same period having the same number of electron shells. It also explains that each column is called a group, with elements in the same group having the same number of valence electrons in their outer shell. The document provides examples of determining the number of shells and valence electrons for various elements based on their period and group.