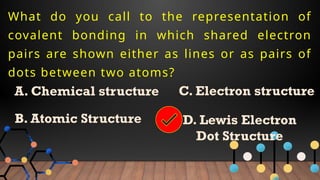
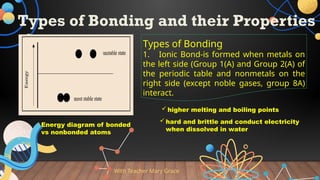
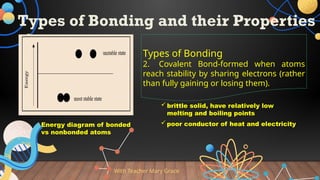
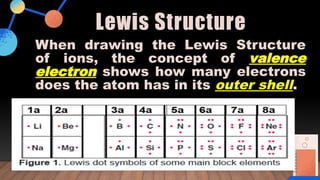
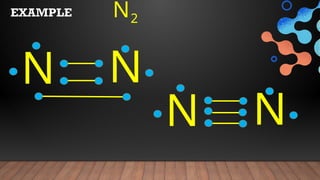
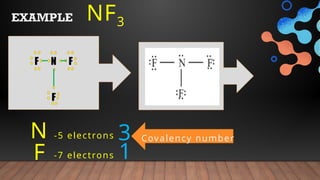
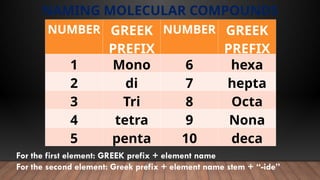
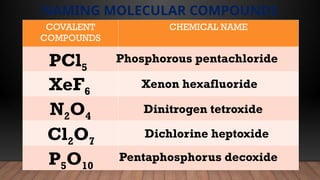
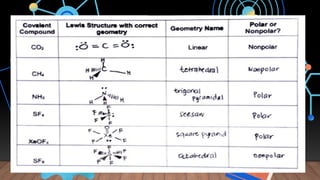
The document provides a comprehensive overview of Lewis structures, bond formation, and types of chemical bonding, including ionic and covalent bonds. It outlines key concepts such as the octet rule, electron configuration, and the steps involved in drawing Lewis structures, along with naming conventions for molecular compounds. Additionally, it includes examples of bonds and properties of bonding types, enhancing understanding of molecular chemistry.