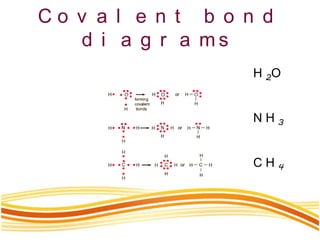
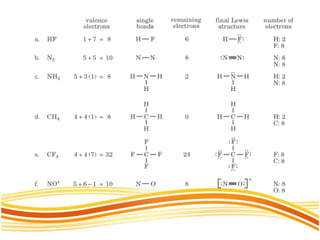
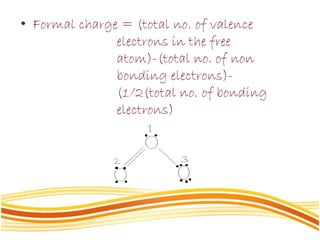
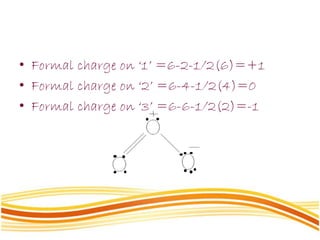
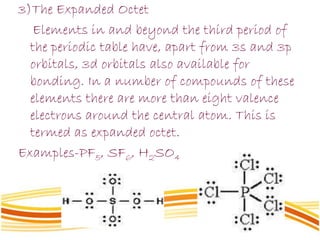
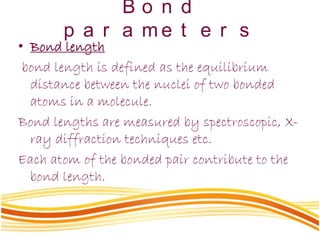
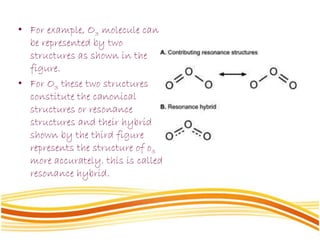
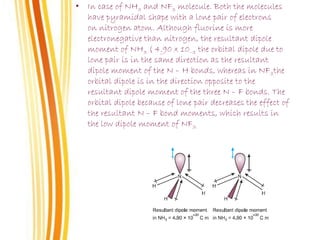
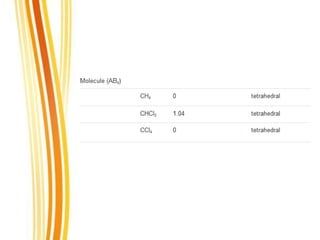
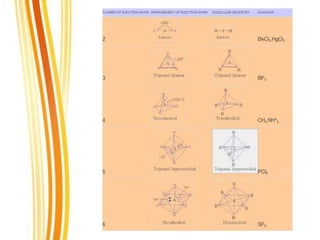
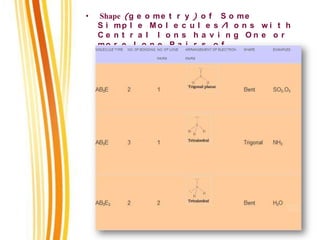
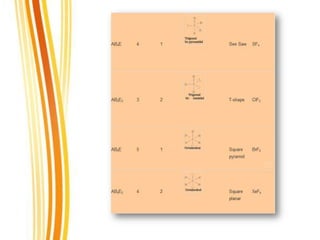
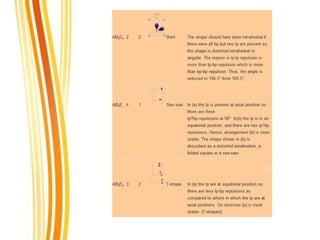
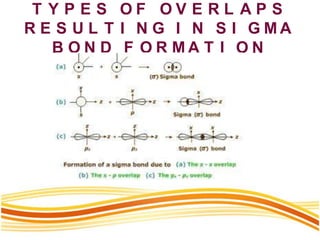
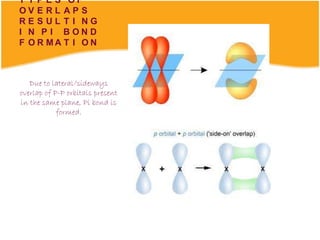
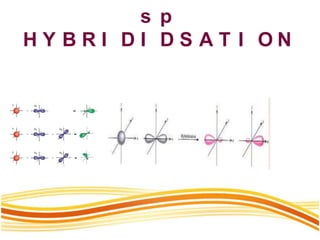
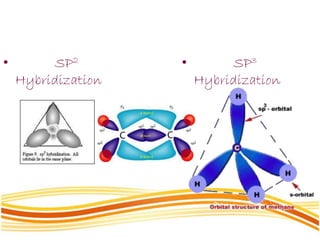
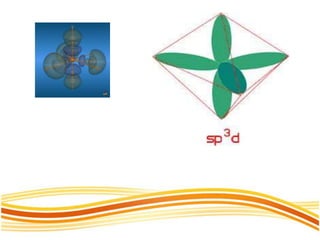
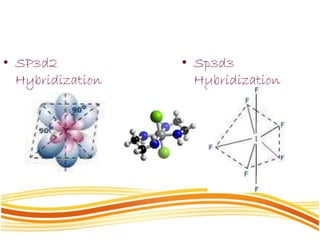
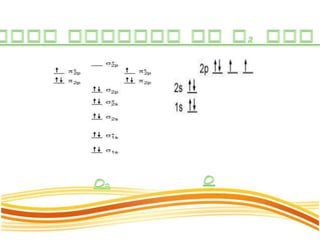
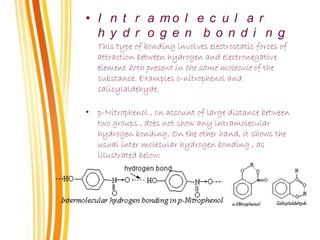
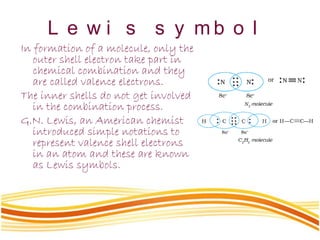
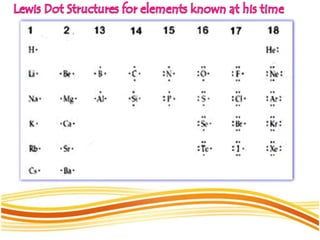
Lewis and Kossel were the first to successfully explain chemical bonding in terms of electrons. They proposed that atoms bond to achieve stable electron configurations like noble gases. Lewis pictured atoms as a positively charged nucleus surrounded by 8 electrons. Atoms bond by transferring or sharing electrons to achieve stable octets. Lewis symbols were introduced to represent valence electrons. Covalent bonds form when atoms share valence electrons in order to gain full outer shells. Resonance structures represent situations where no single Lewis structure adequately describes the molecule.





![Example
• Na Na+ + e-
[N e ] 3s 1 [N e ]
• Cl + e- Cl-
[N e } 3s 2 3p 5 [N e ] 3s 2 3p 6 o r [A r ]
=> Na+ + Cl- NaCl](https://image.slidesharecdn.com/chemistry-130513020109-phpapp02-181123092700/85/Chemistry-molecular-structure-9-320.jpg)

![R e c a l l : E l e c t r o n s a r e
d i v i d e d b e t w e e n c o r e
a n d v a l e n c e
e l e c t r o n s .
A T O M c o r e
v a l e n c e
N a 1s 2 2s 2 2p 6 3s 1 [N e ]
3s 1
B r [A r ] 3d 10 4s 2 4p 5 [A r ] 3d 10
4s 2 4p 5
C o v a l e n t
B o n d i n g
Covalent bond is the sharing of the VALENCE
ELECTRONS of each atom in a bond
Br Br](https://image.slidesharecdn.com/chemistry-130513020109-phpapp02-181123092700/85/Chemistry-molecular-structure-11-320.jpg)