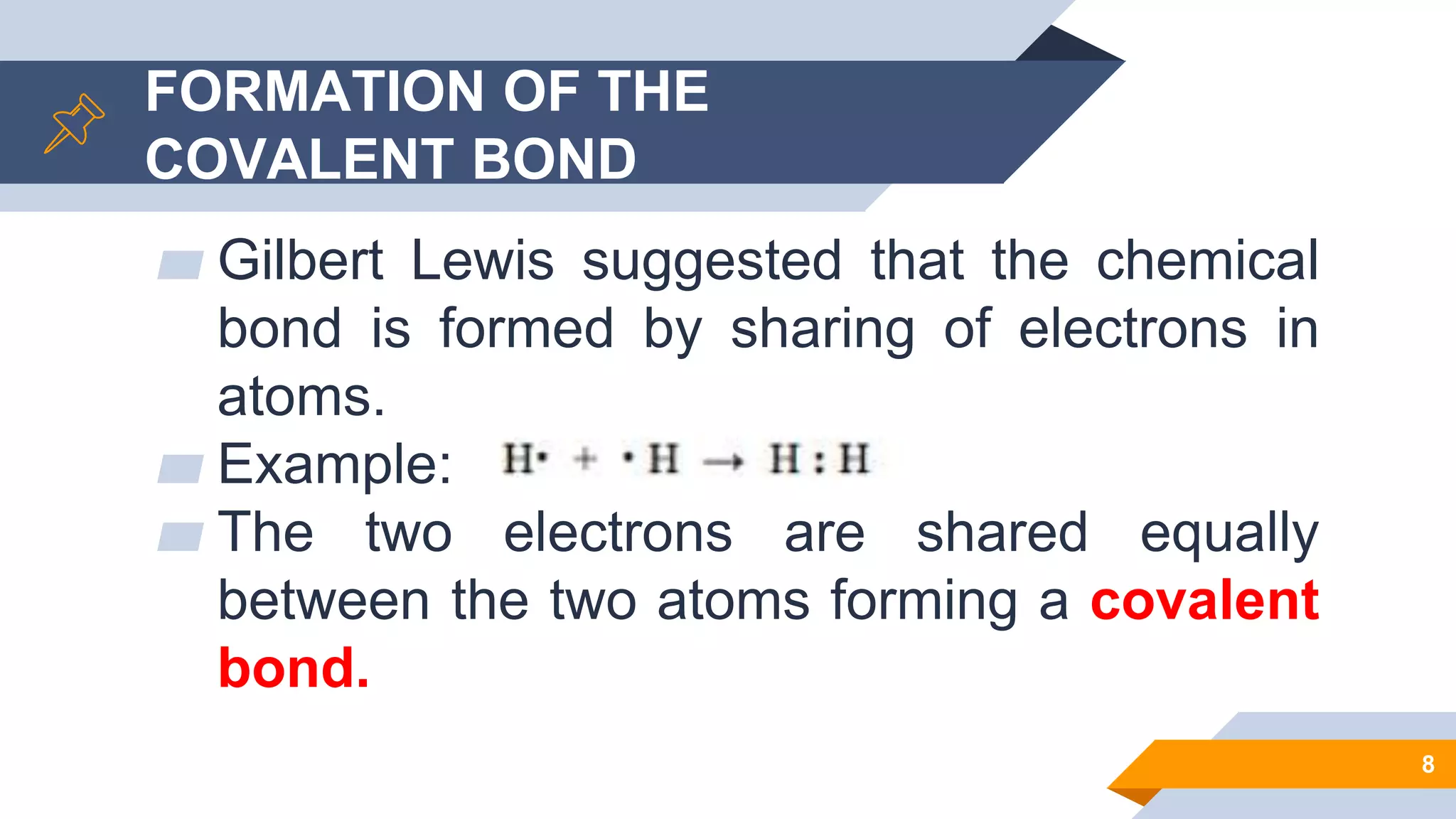
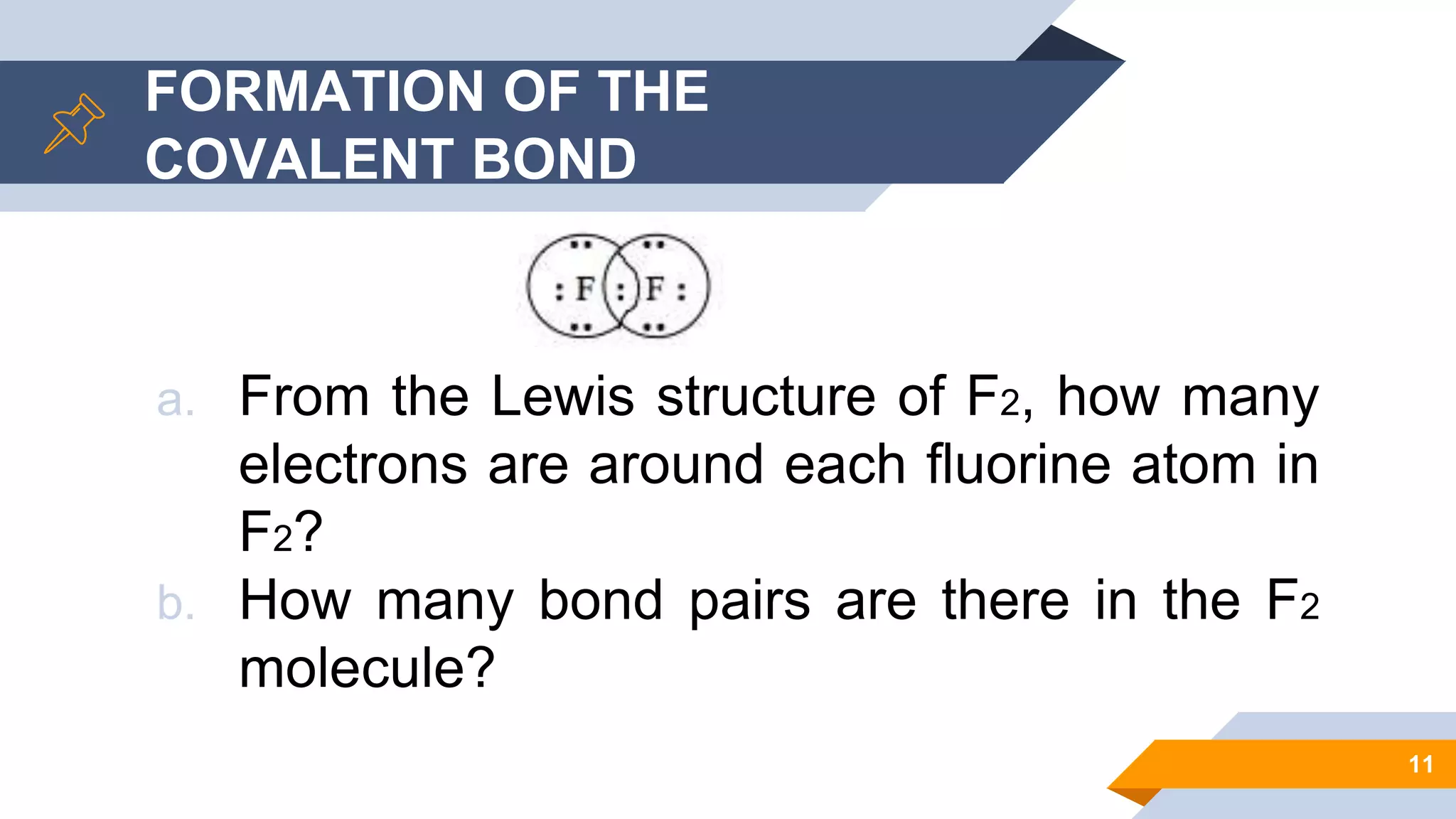
This document discusses covalent bonds and Lewis structures. It begins by listing learning objectives related to covalent bonding concepts. It then defines key terms like Lewis structure, covalent bond, and electronegativity. The document goes on to explain the formation of covalent bonds via electron sharing. It discusses exceptions to the octet rule. Guidelines are provided for drawing Lewis structures, including dealing with resonance. Various examples of Lewis structures are worked through. The document concludes with a review of naming covalent compounds.