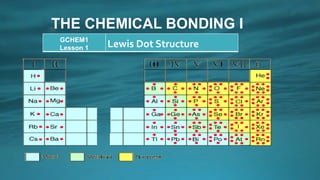
The document discusses Lewis dot structures and how they are used to represent valence electrons in atoms and molecules. It provides examples of writing Lewis dot structures according to the octet rule, which states that atoms are most stable when their valence shells are filled with eight electrons. It also discusses how covalent bonds are formed by the sharing of valence electrons between atoms to achieve stable octet configurations. Key points covered include how to draw Lewis dot structures, count valence electrons, and distribute electrons to atoms following the octet rule.