Embed presentation

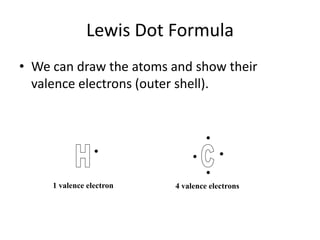
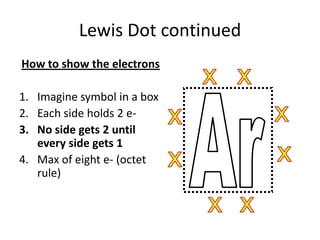
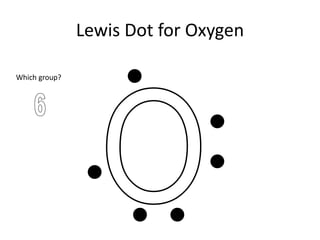


This document discusses Lewis dot structures, also known as electron dot structures or Lewis dot formulas. Lewis dot structures show atoms and their valence electrons using dots arranged around the atomic symbols. Each side of the atomic symbol box can hold two electrons, following the octet rule where atoms gain a full outer shell of eight electrons through bonding. The document provides examples of drawing Lewis dot structures for hydrogen, carbon, argon, and oxygen atoms and encourages practicing drawing structures for chlorine, phosphorus, and neon.




