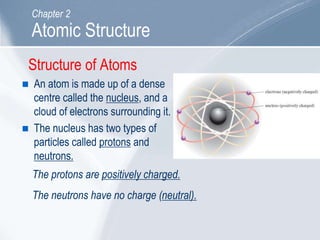
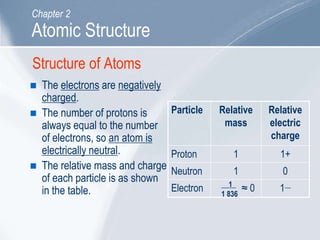
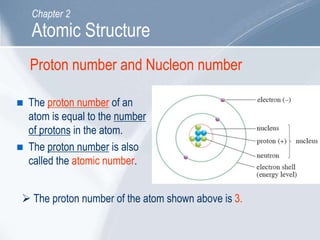
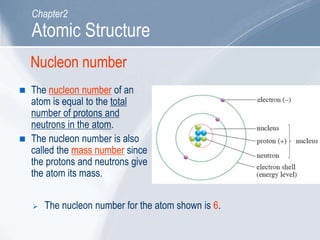
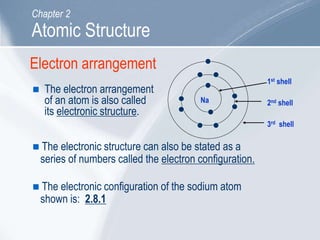
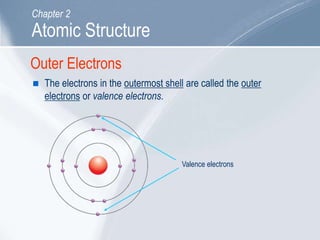
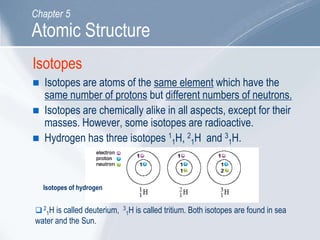
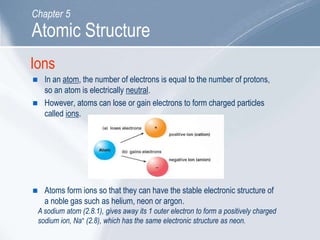
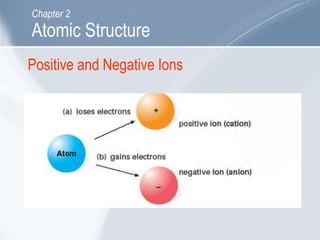
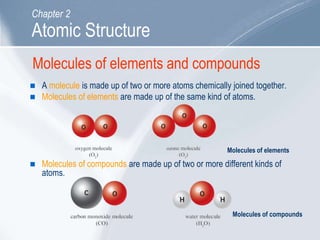
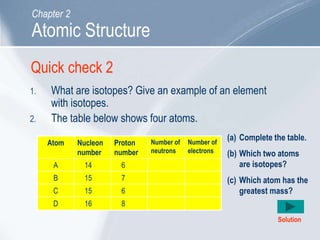
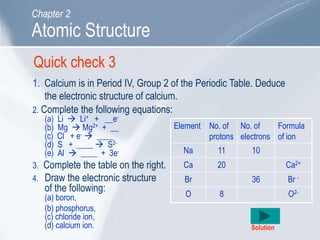
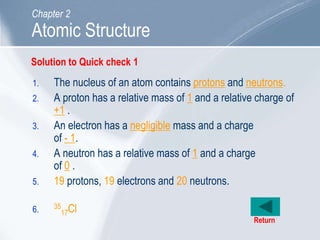
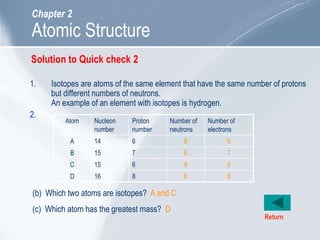
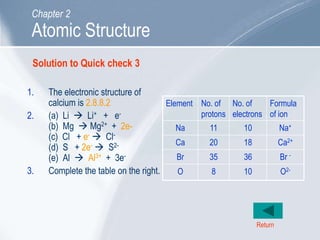
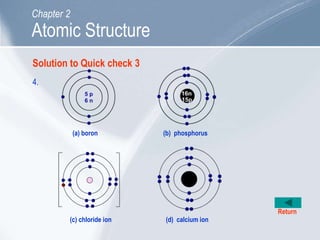
The document outlines key concepts about atomic structure including the structure of atoms with protons, neutrons and electrons, atomic number and mass number, electron configuration, isotopes, ions, and molecules of elements and compounds. It also provides learning outcomes for describing atomic structure and properties as well as interpreting atomic symbols and notations.