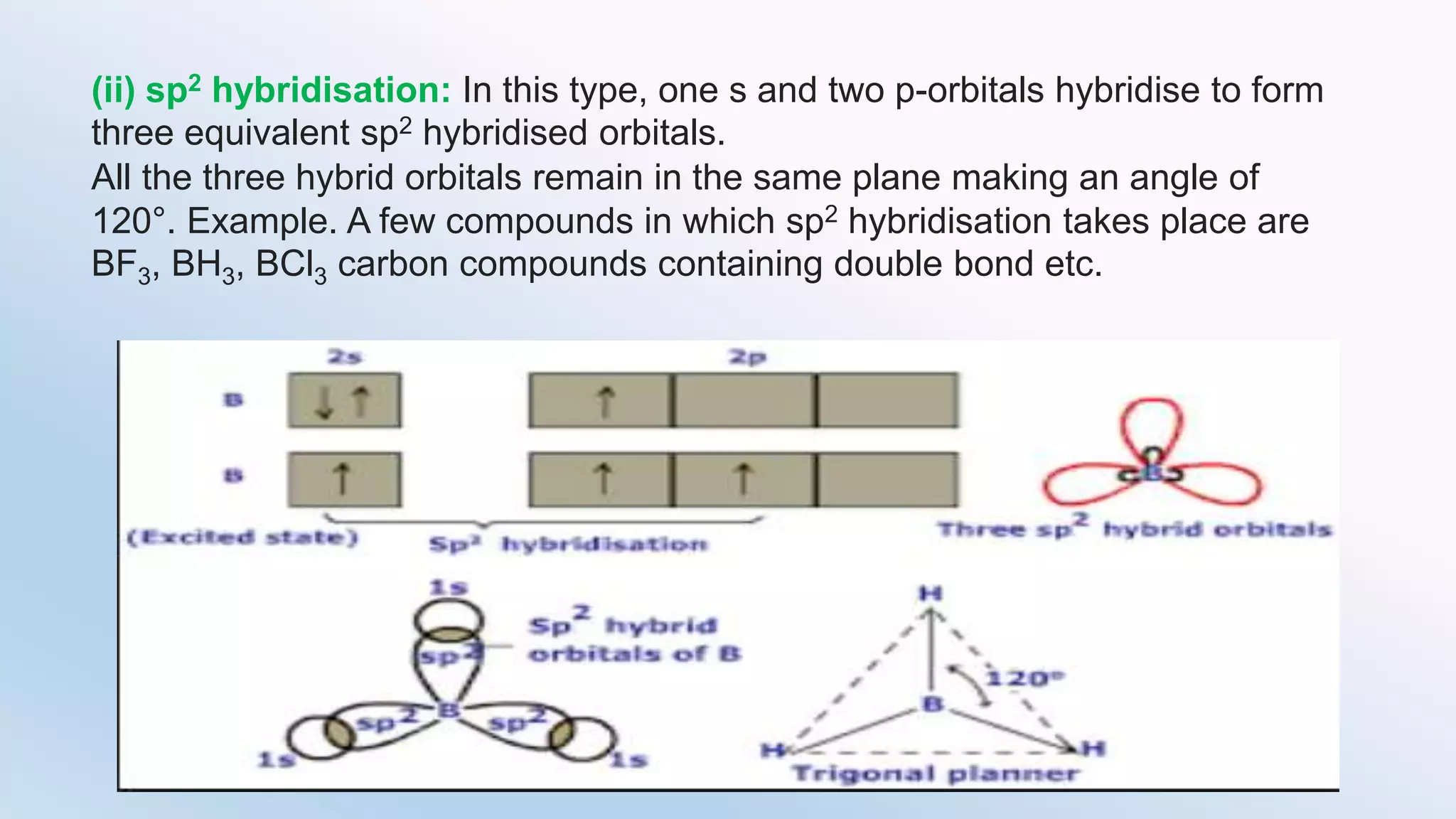
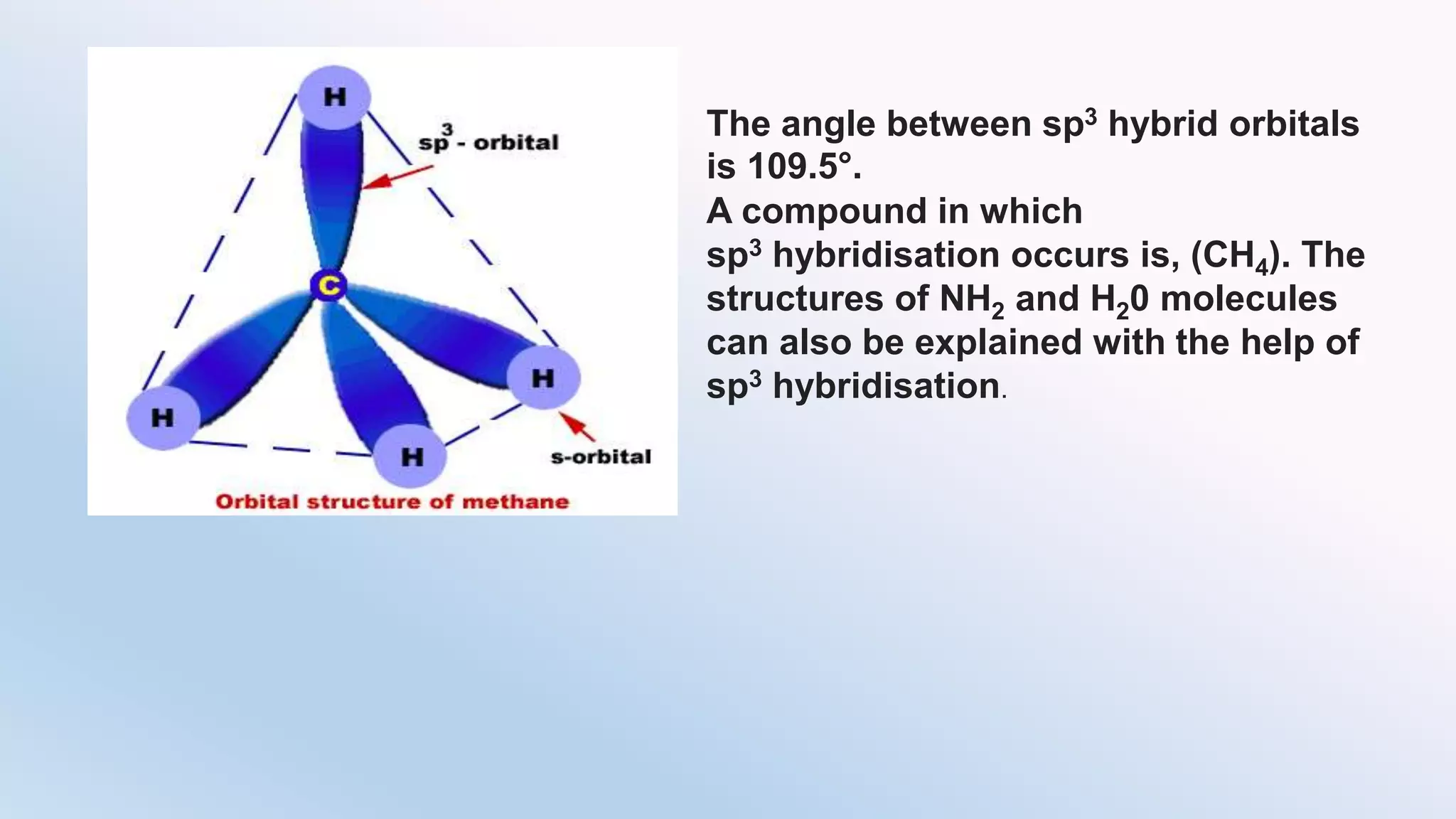
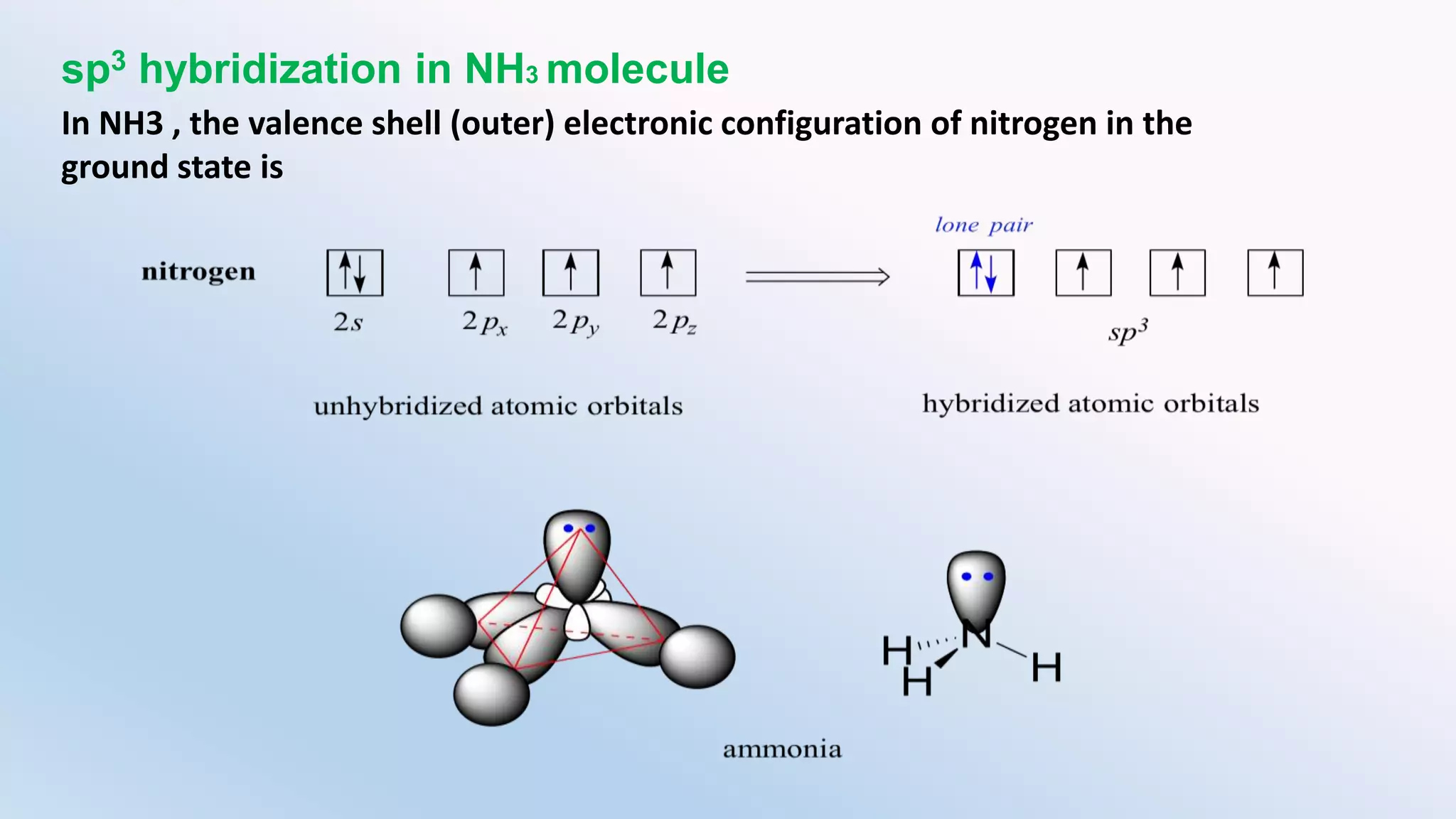
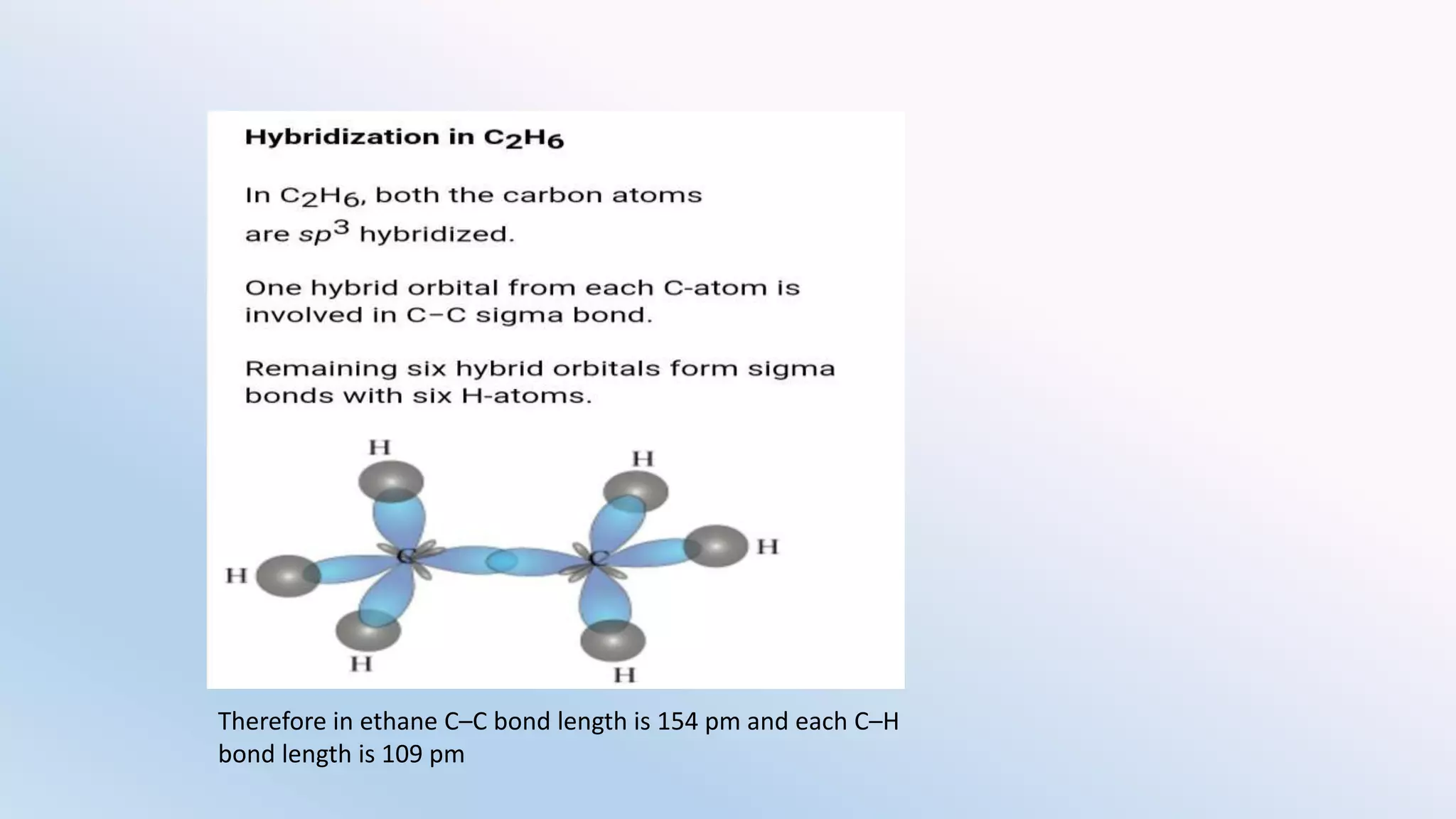
Chemical bonding can occur via ionic bonds or covalent bonds. Ionic bonds form when electrons are transferred from one atom to another, leaving cation and anion. Covalent bonds form when atoms share electrons via overlapping orbitals. The octet rule states that atoms seek to obtain eight electrons in their valence shell. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals for bonding. Common hybridizations include sp, sp2, and sp3 which determine molecular geometry. Bond properties like order, length, energy are influenced by hybridization.
























![2. Pi Bond (π bond)
It is formed by the sidewise or lateral overlapping between p-
atomic orbitals [pop side by side or lateral overlapping]
π bond is a weaker bond than σ bond.
Strength of Sigma and pf Bonds
Sigma bond (σ bond) is formed by the axial overlapping of the atomic
orbitals while the π-bond is formed by side wise overlapping. Since
axial overlapping is greater as compared to side wise. Thus, the sigma
bond is said to be stronger bond in comparison to a π-bond.
Distinction between sigma and n bonds](https://image.slidesharecdn.com/chemicalbonding-200729183835/75/CHEMICAL-BONDING-AND-MOLECULAR-STRUCTURE-29-2048.jpg)