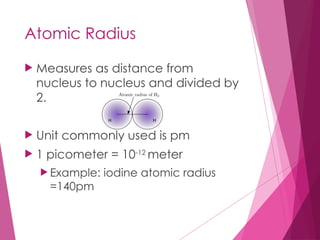
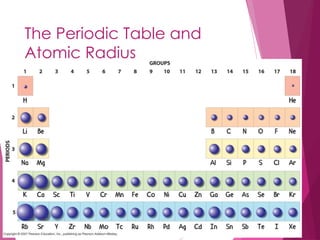
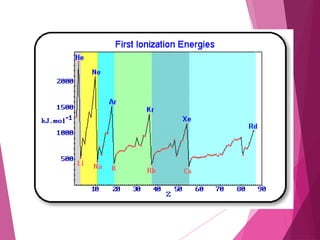
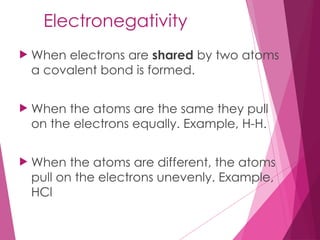
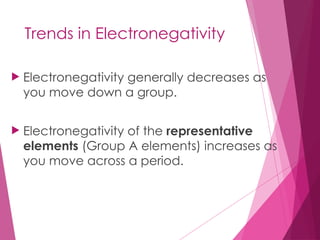
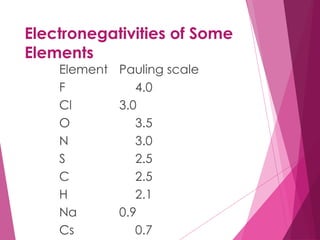
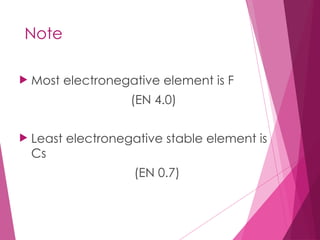
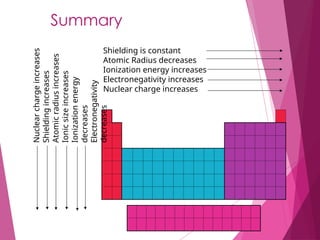
The document outlines key periodic trends in chemistry, focusing on atomic size, ionization energy, and electronegativity. It explains how these properties change across periods and down groups, emphasizing the influence of nuclear charge and electron shielding. The summary concludes that while atomic size and ionization energy generally decrease and increase respectively across a period, electronegativity increases in this direction but decreases down a group.