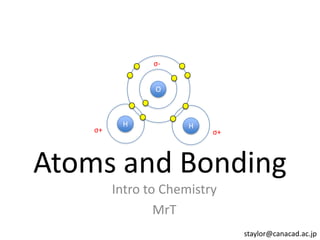
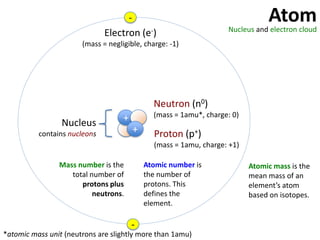
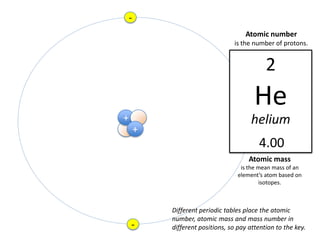
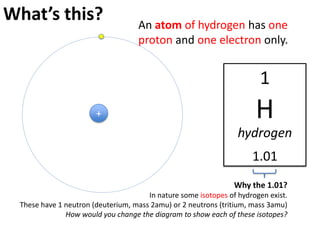
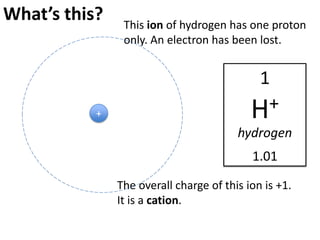
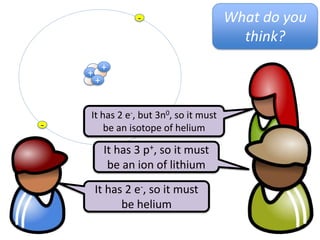
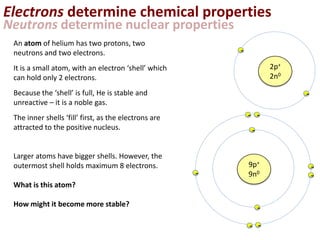
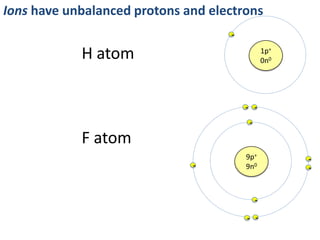
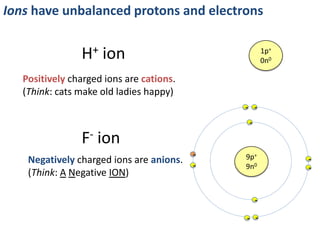
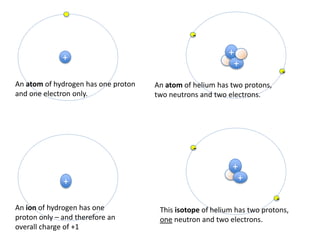
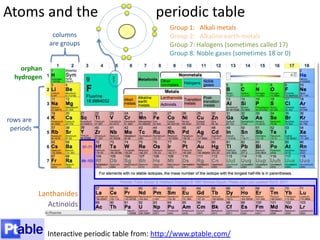
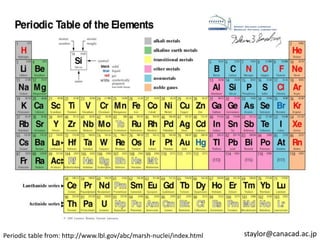
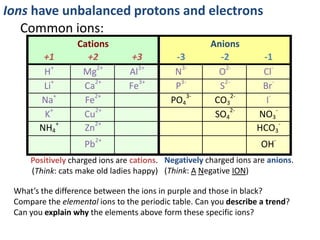
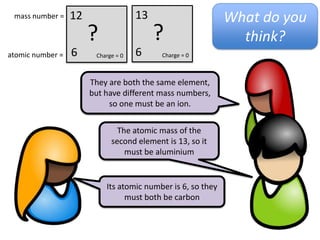
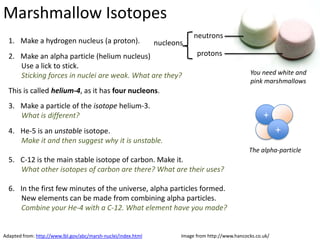
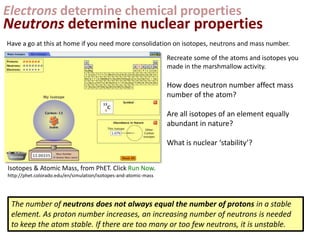
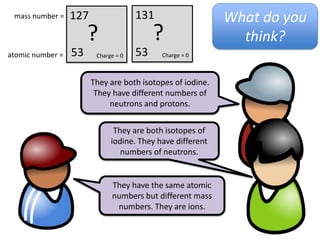
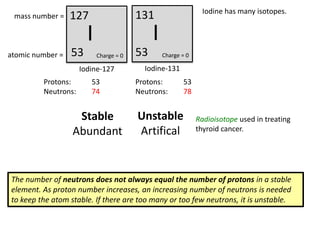
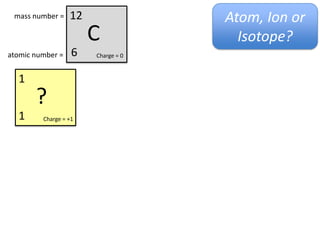
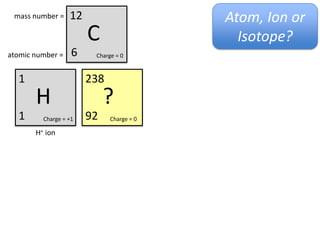
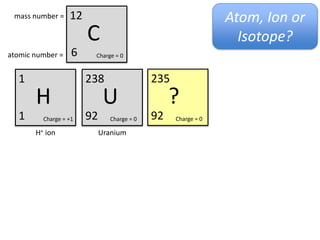
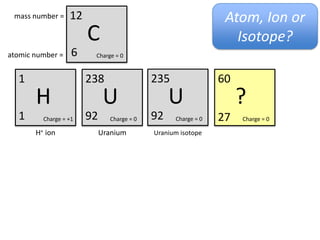
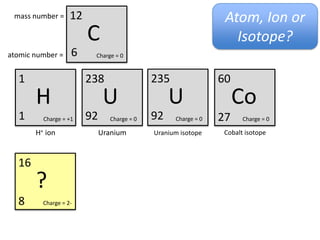
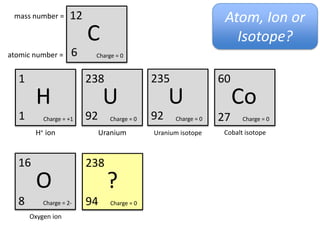
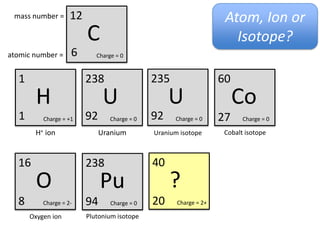
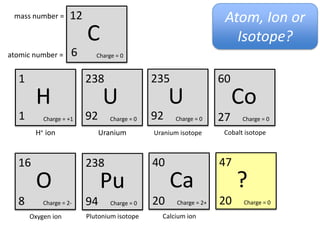
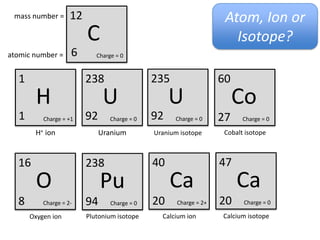
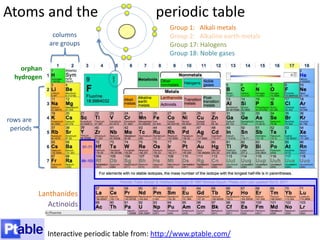
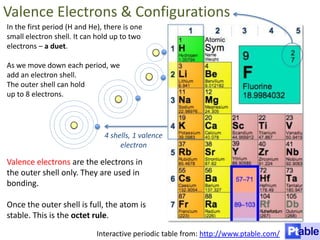
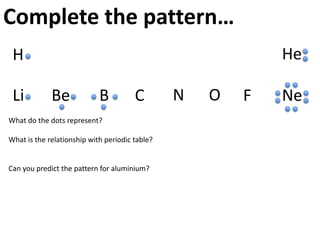
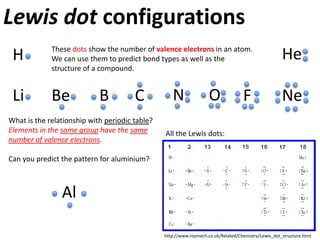
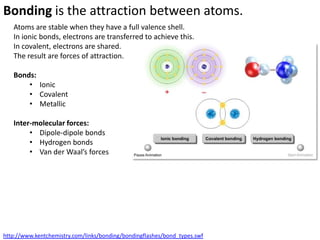
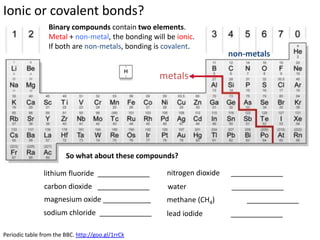
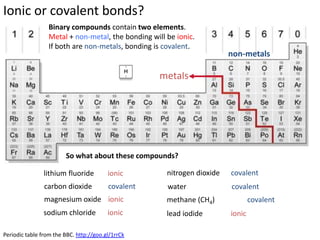
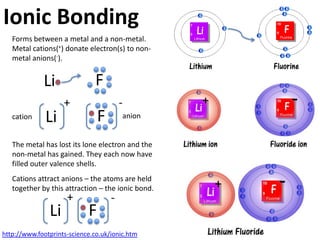
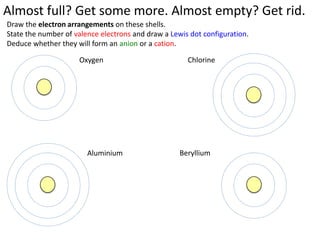
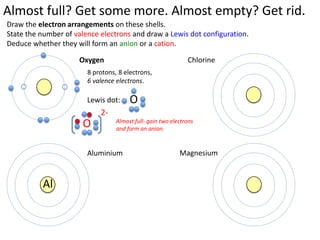
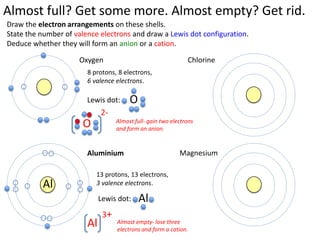
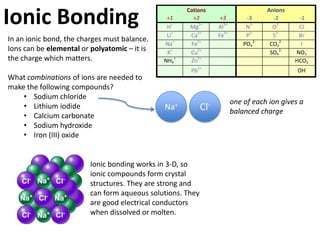
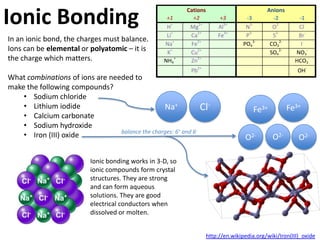
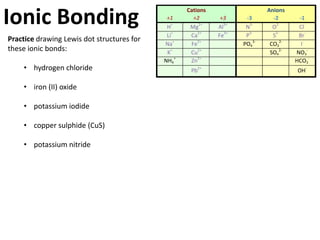
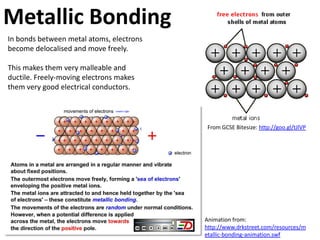
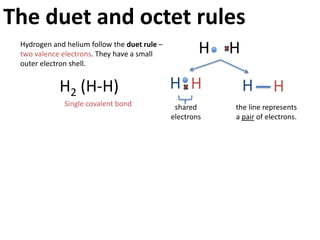
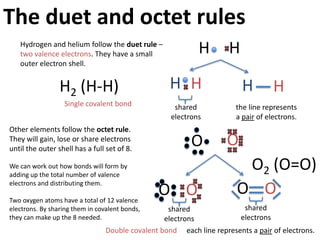
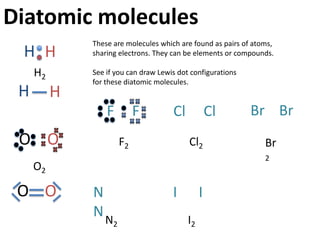
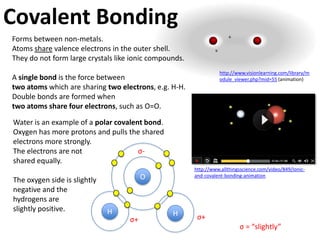
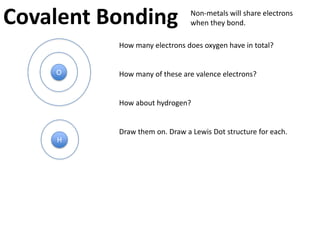
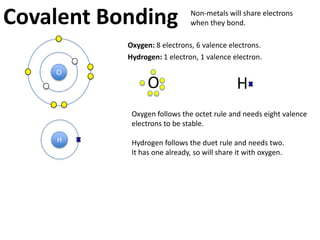
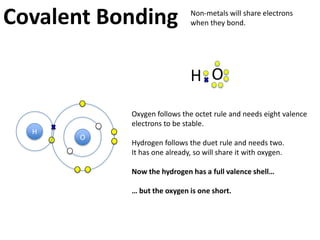
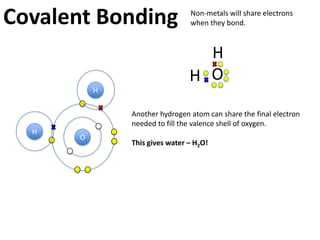
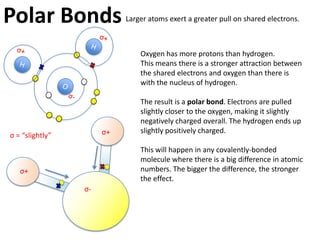
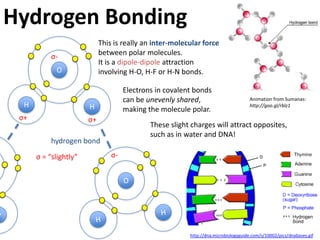
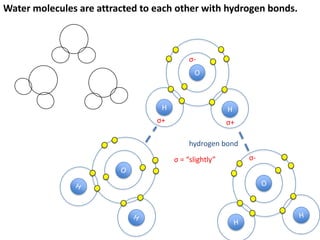
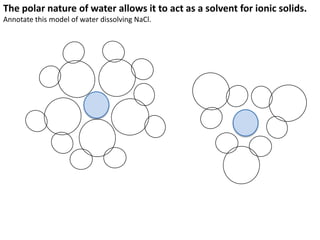
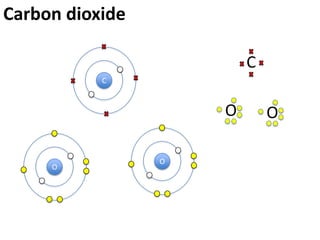
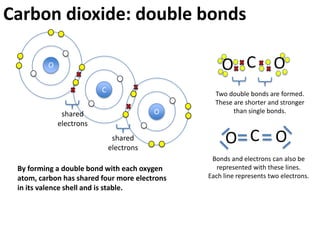
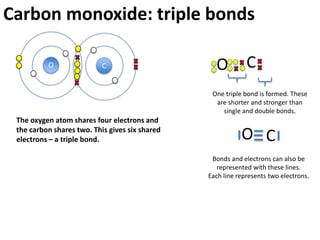
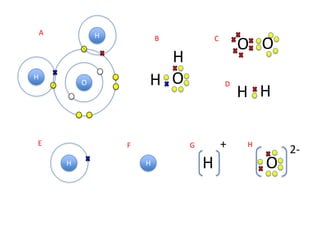
This represents two isotopes of carbon:
1. Carbon-12: Has 6 protons and 6 neutrons. Neutral charge.
2. Carbon-13: Has 6 protons and 7 neutrons. Also neutral charge.
Carbon-13 is a stable isotope of carbon. The increased neutron number makes it an isotope. Both have 6 protons so they are the element carbon.