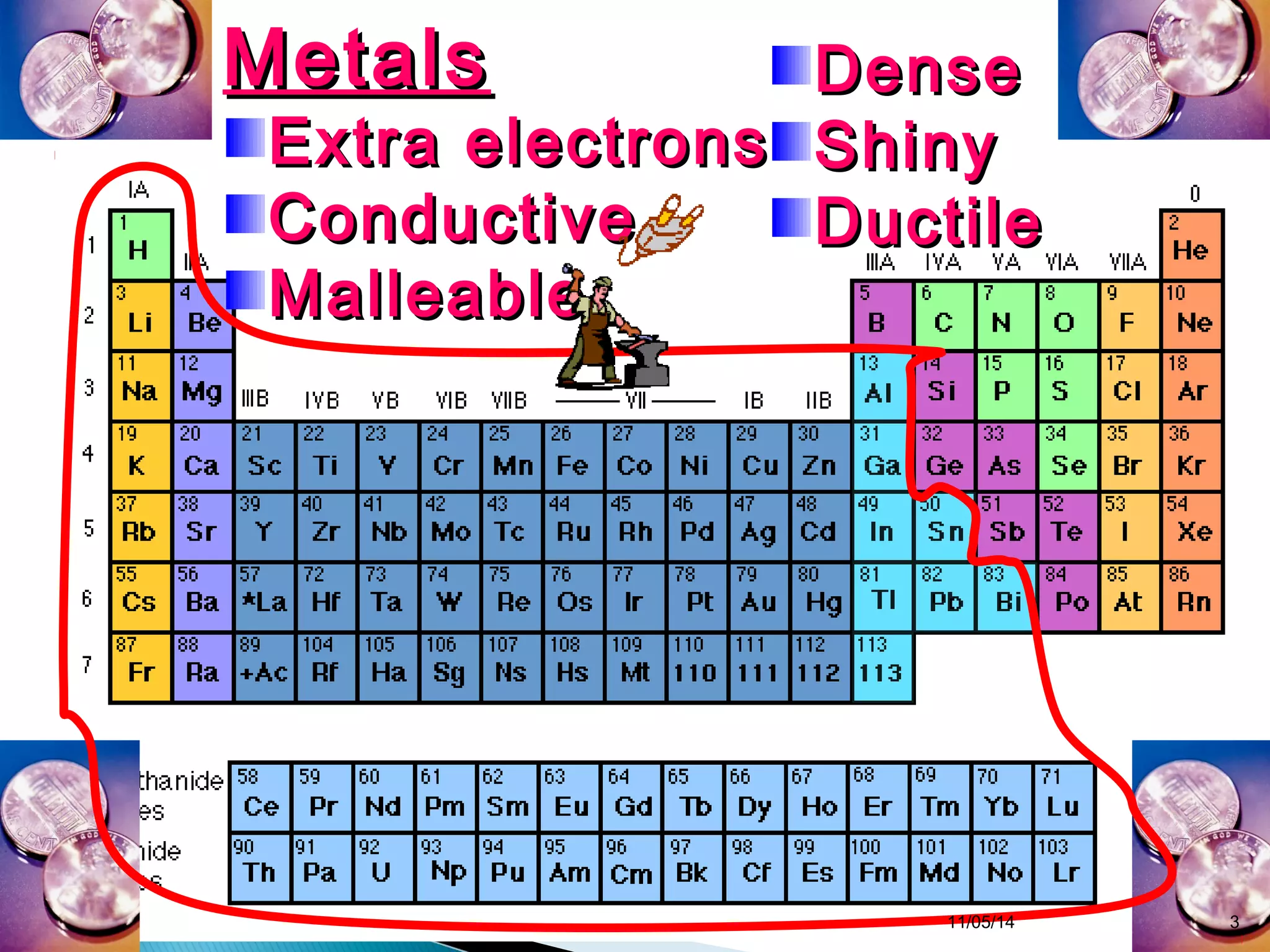
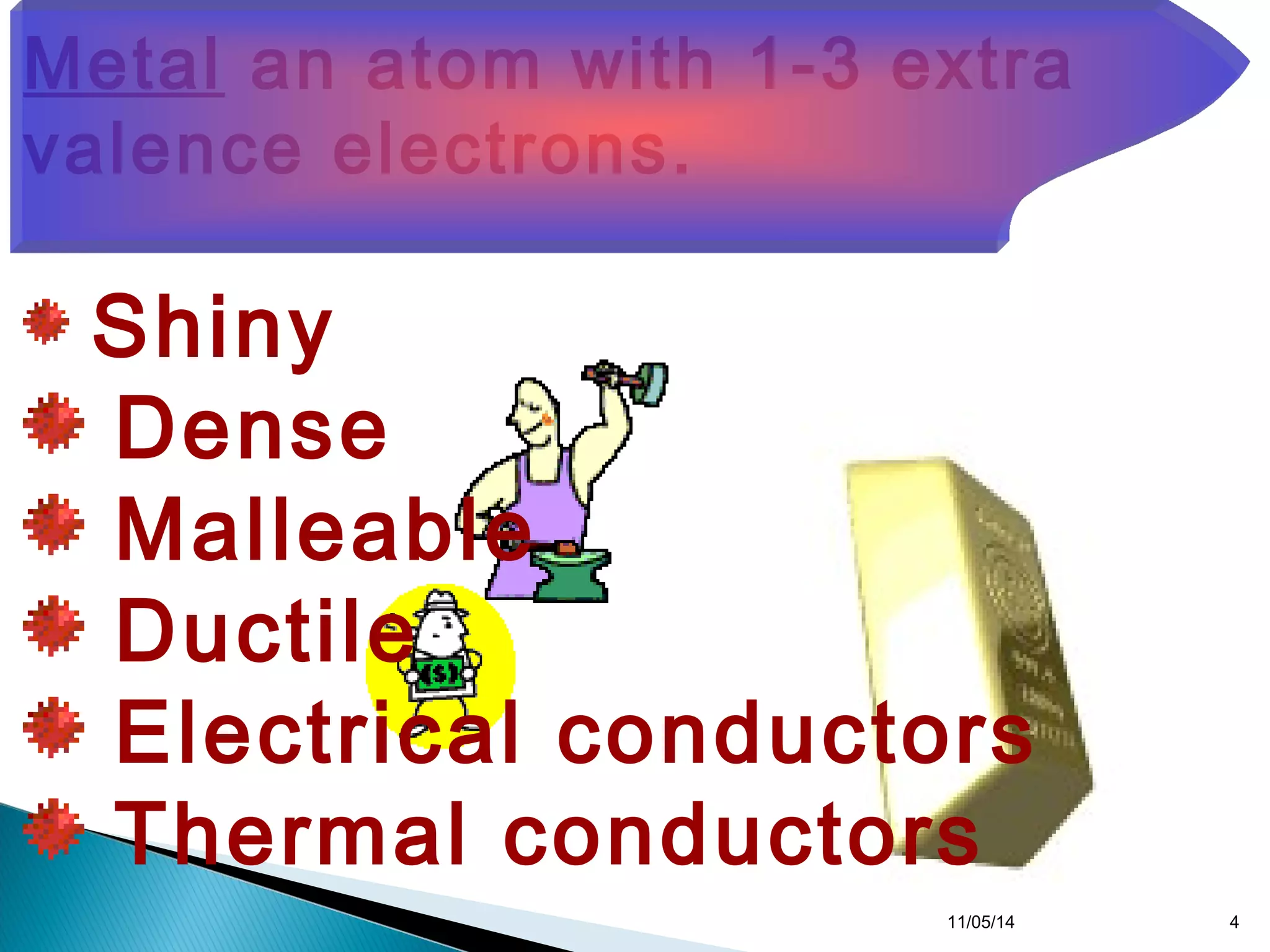
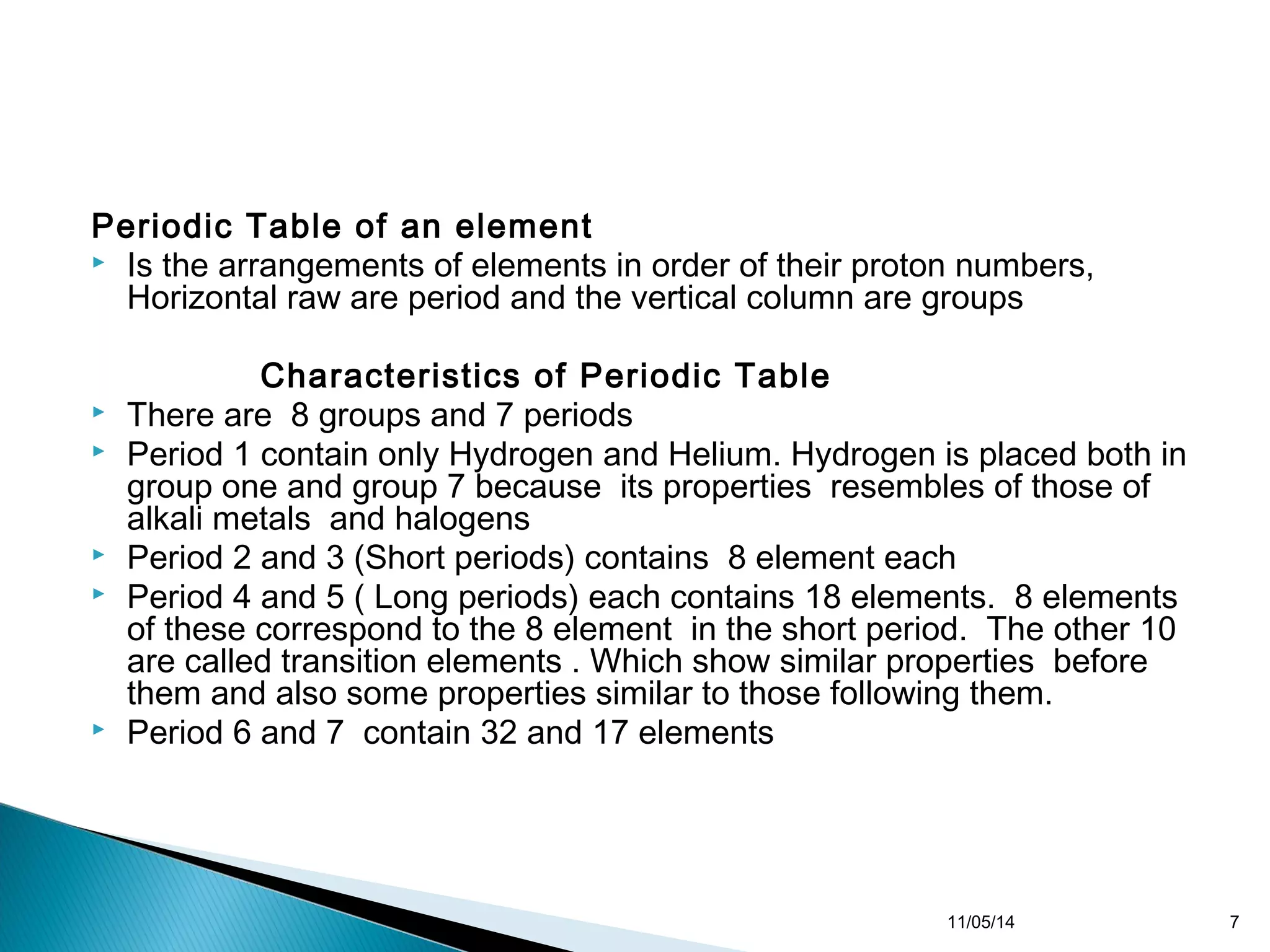
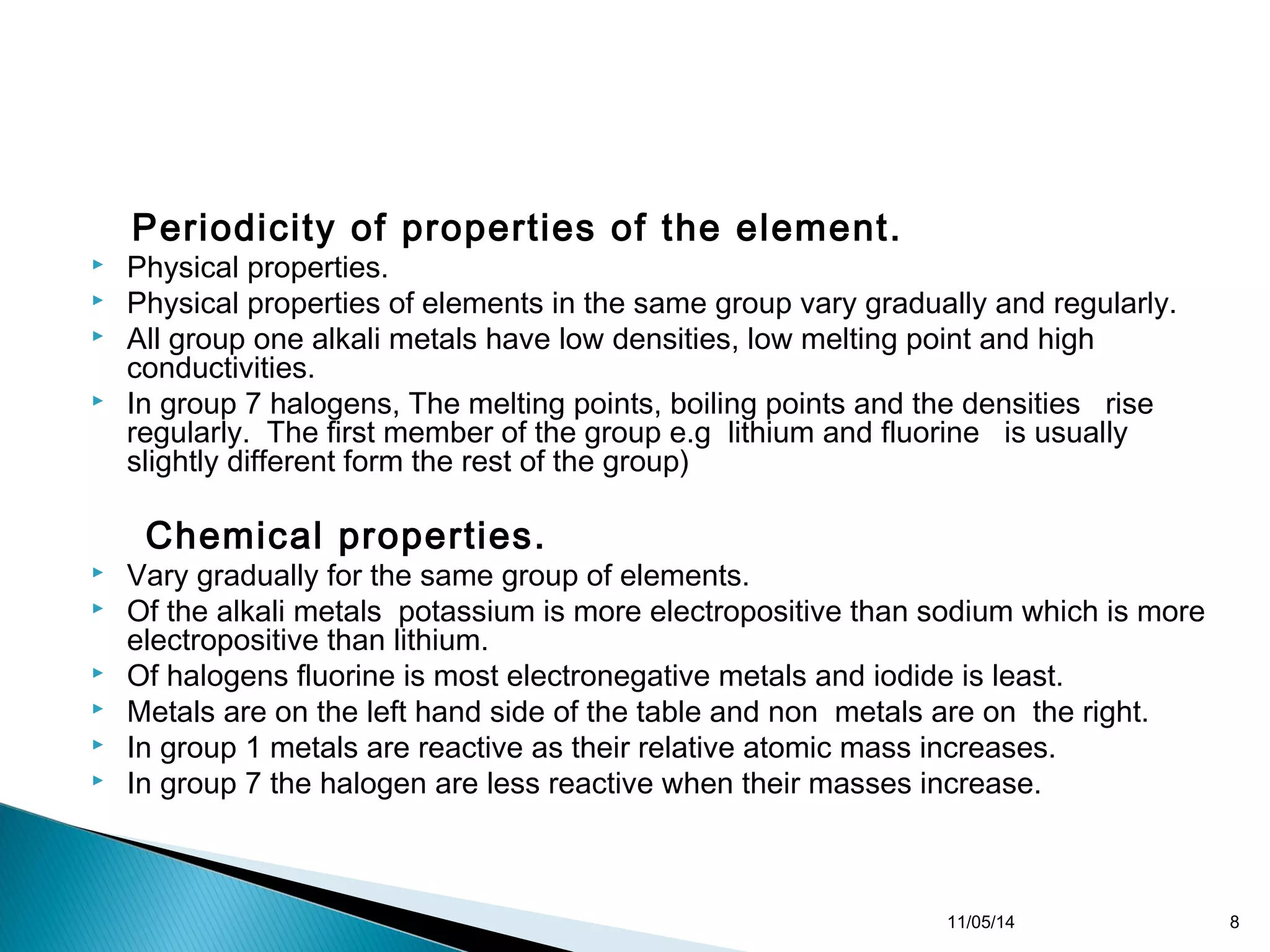
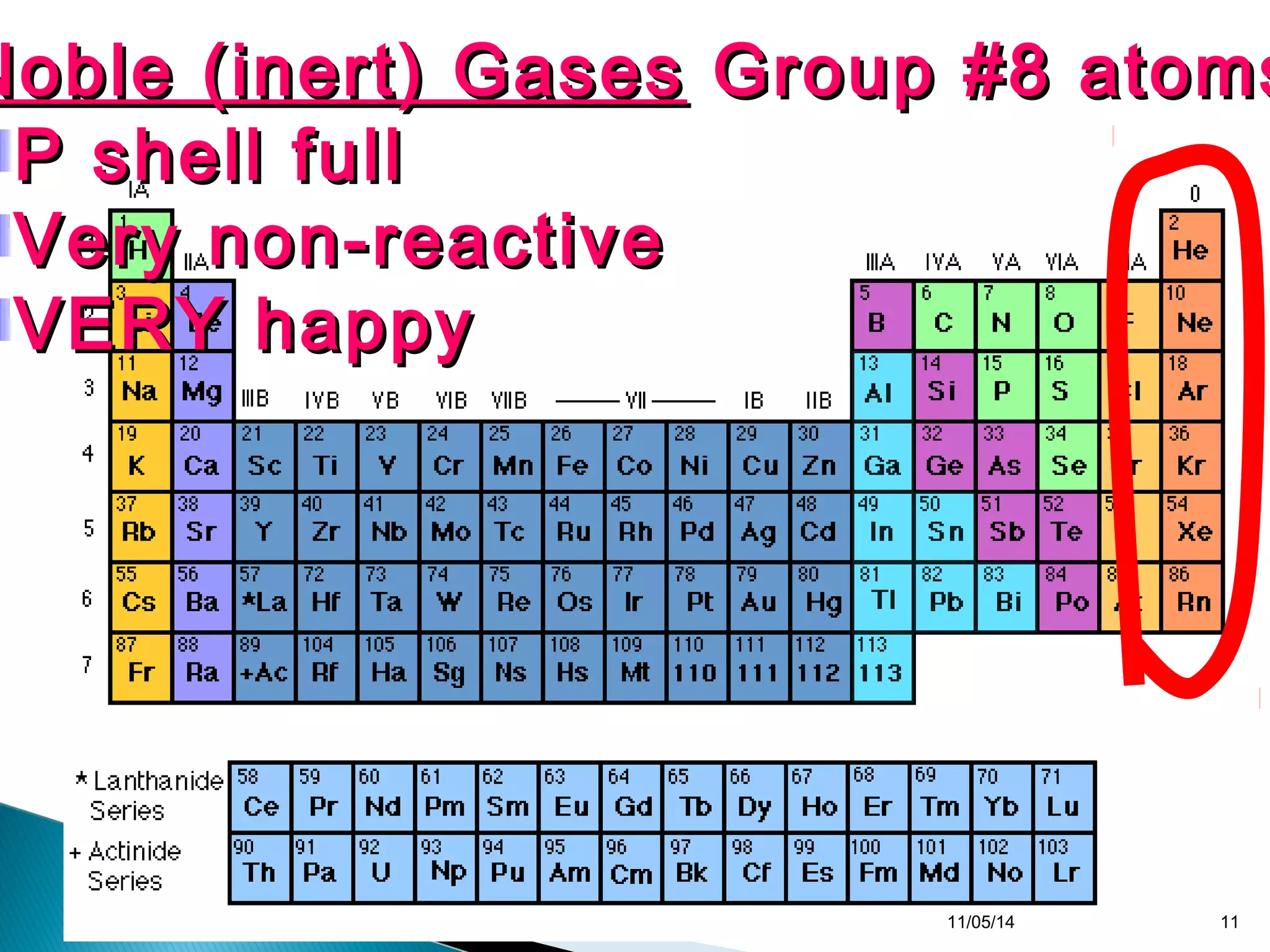
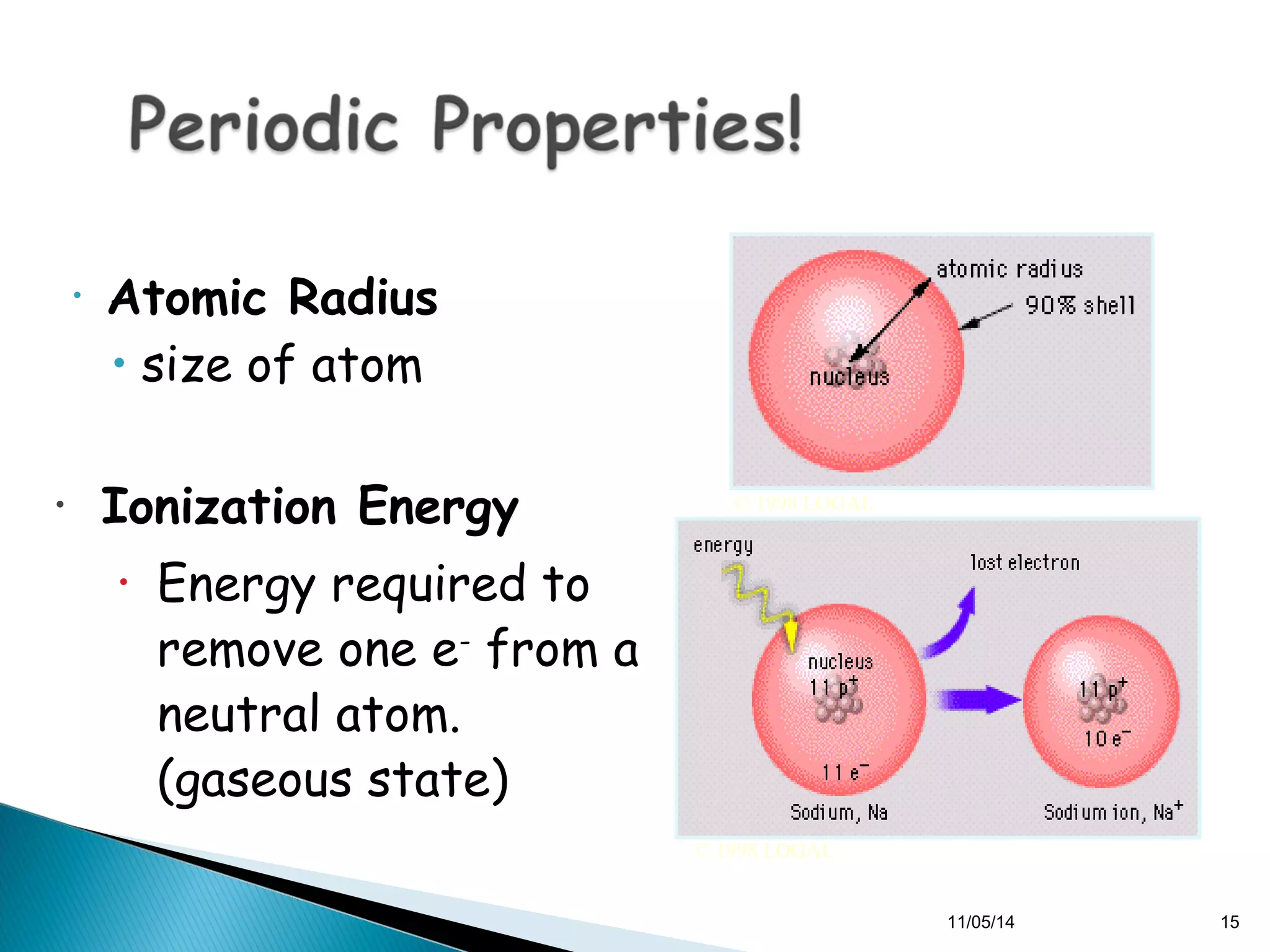
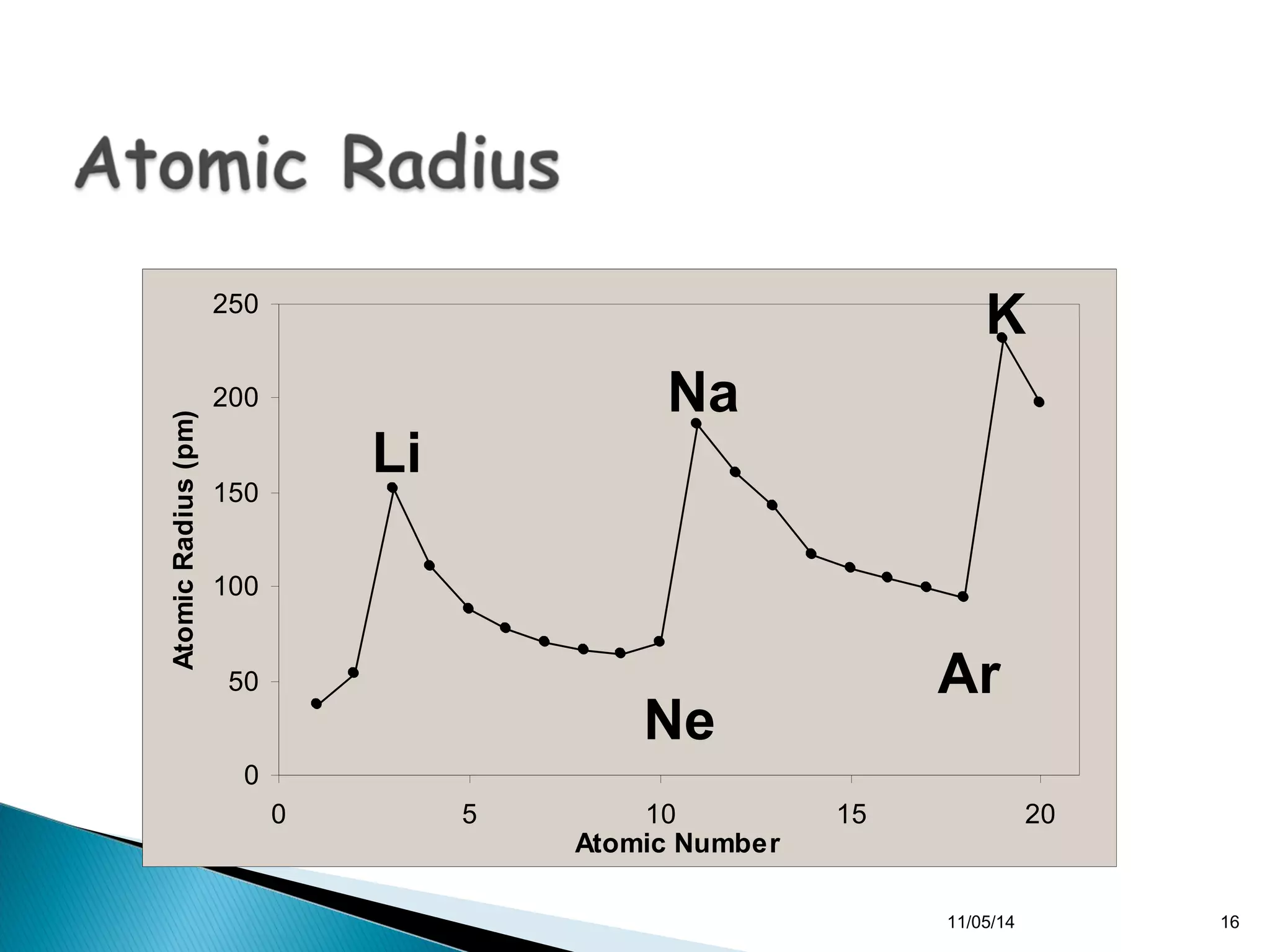
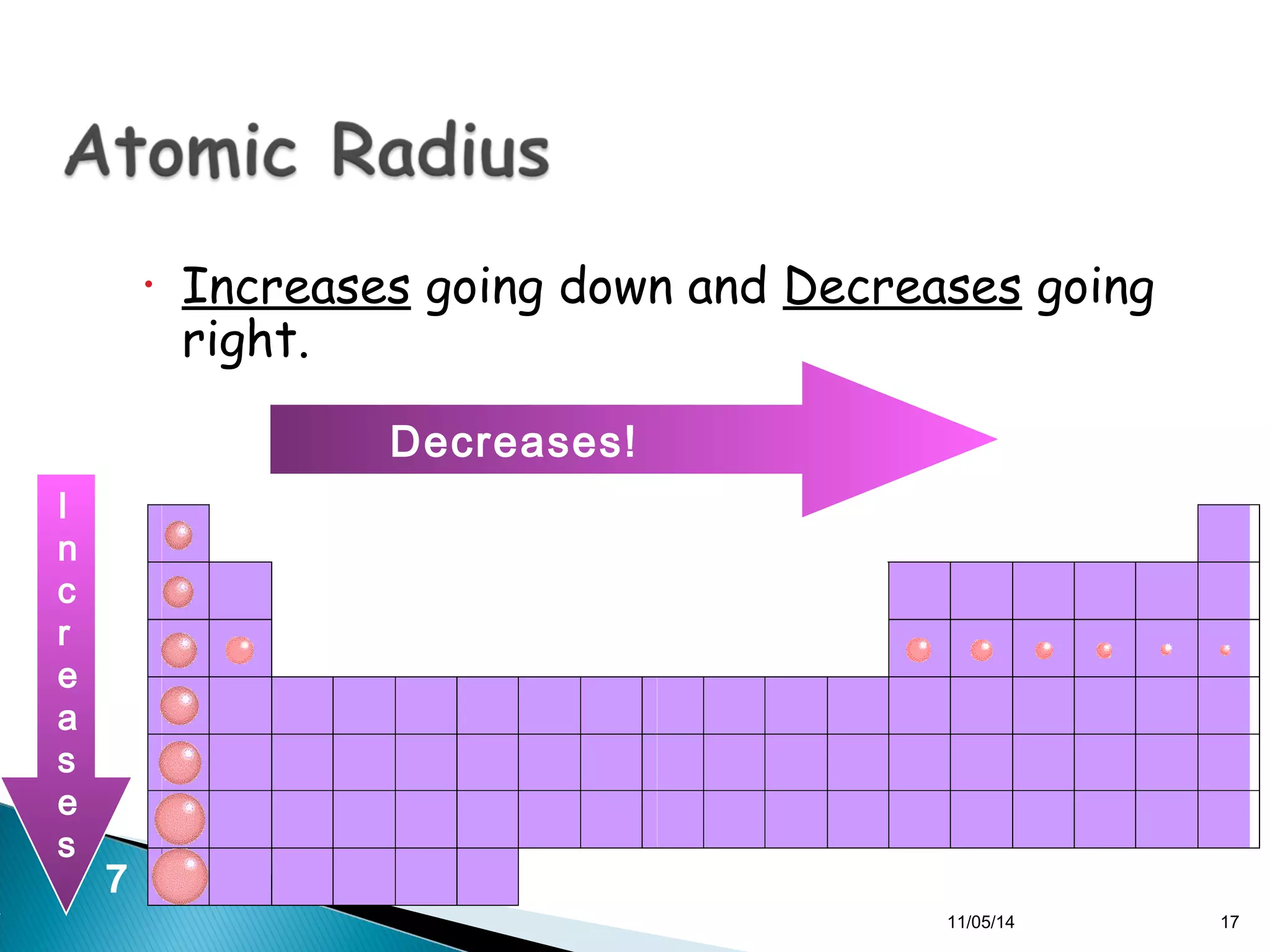
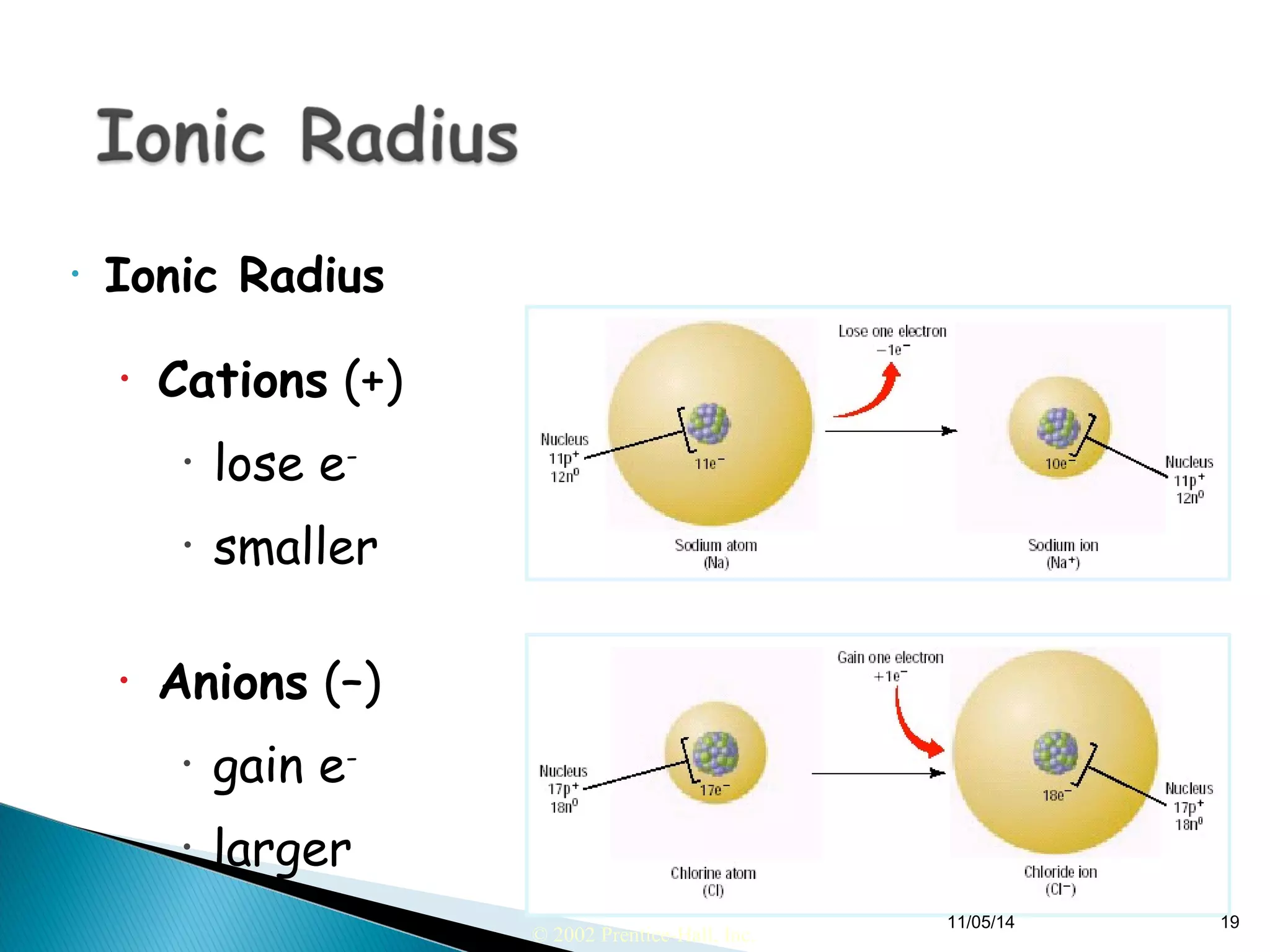
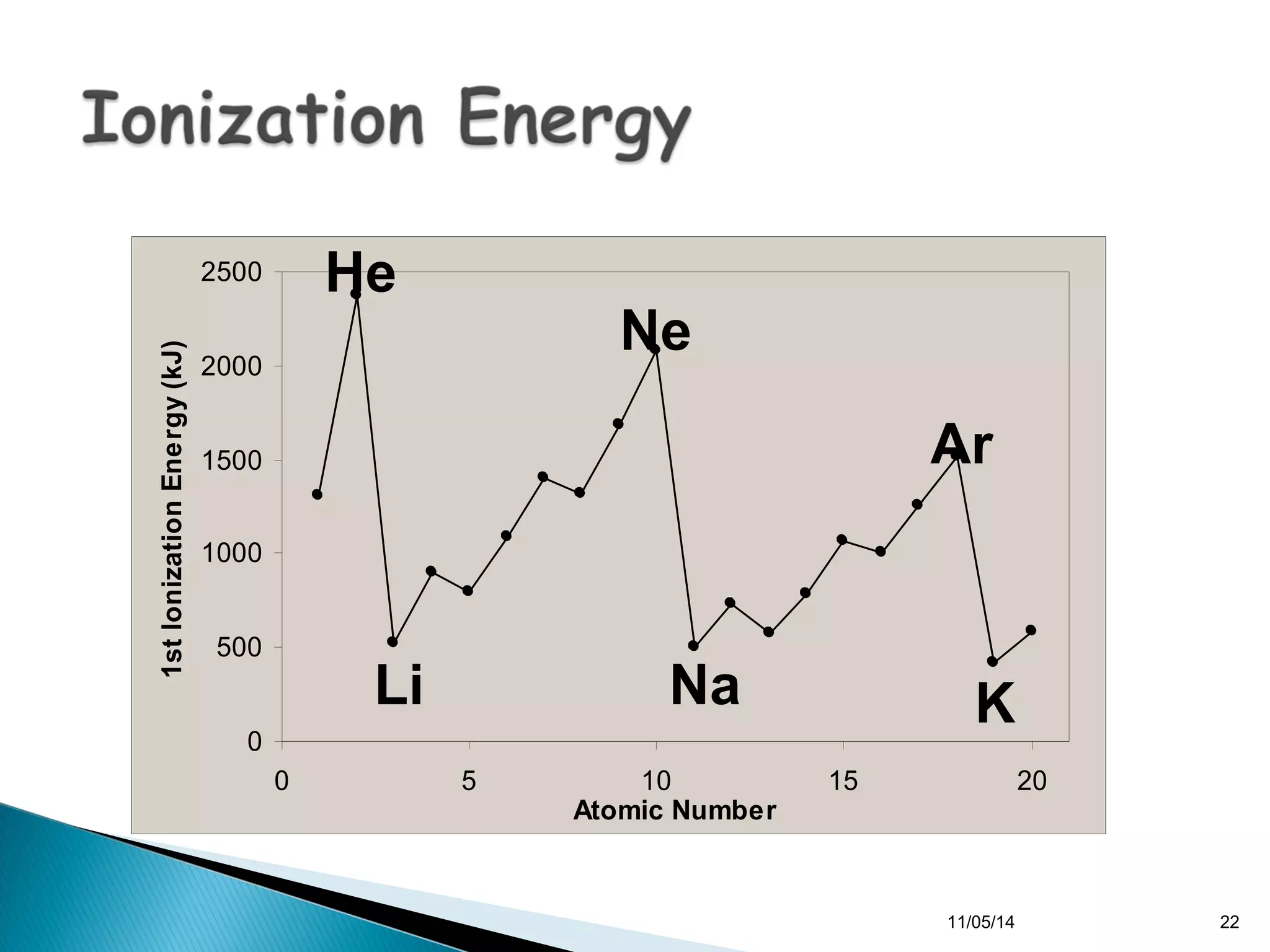
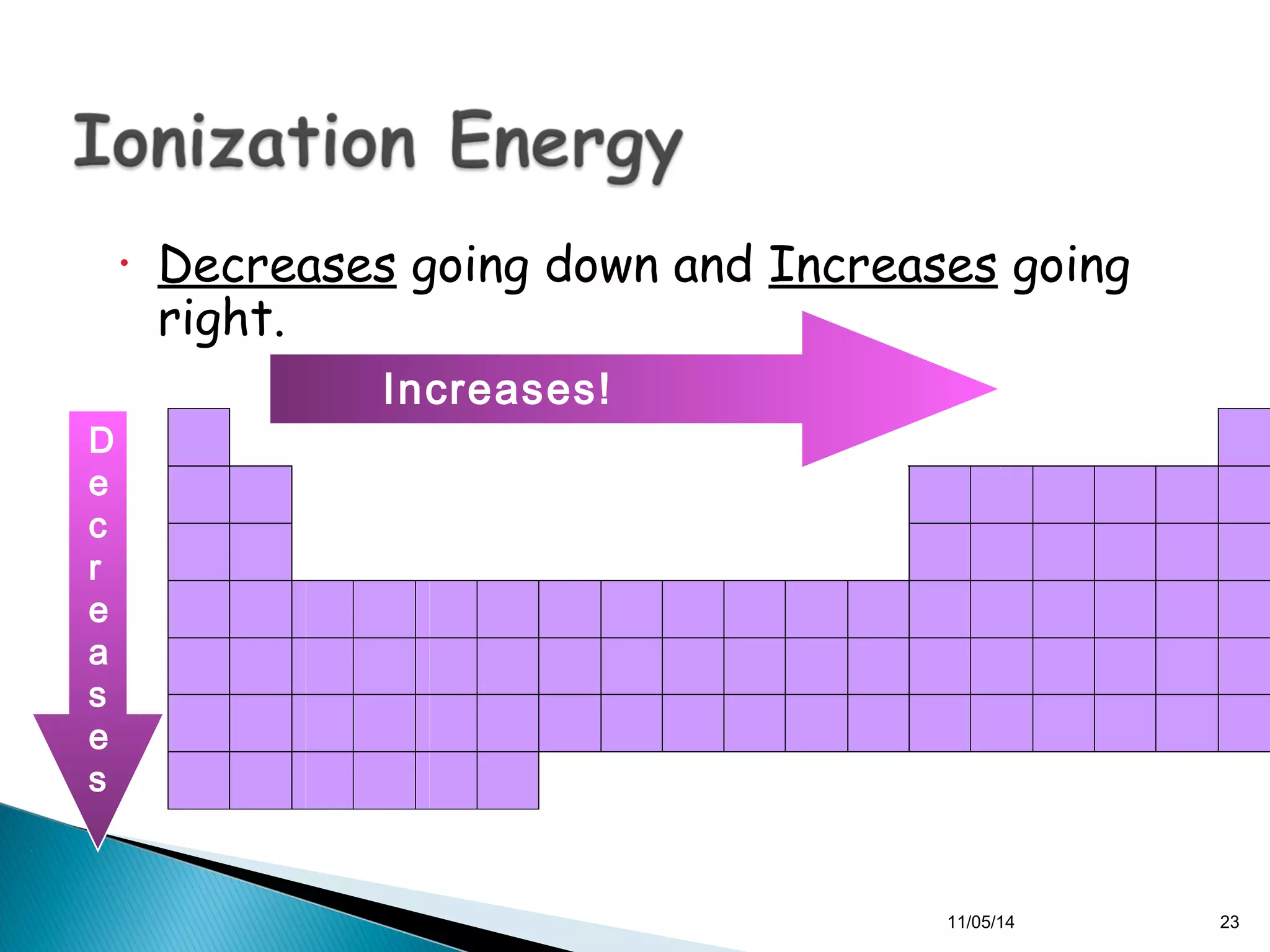
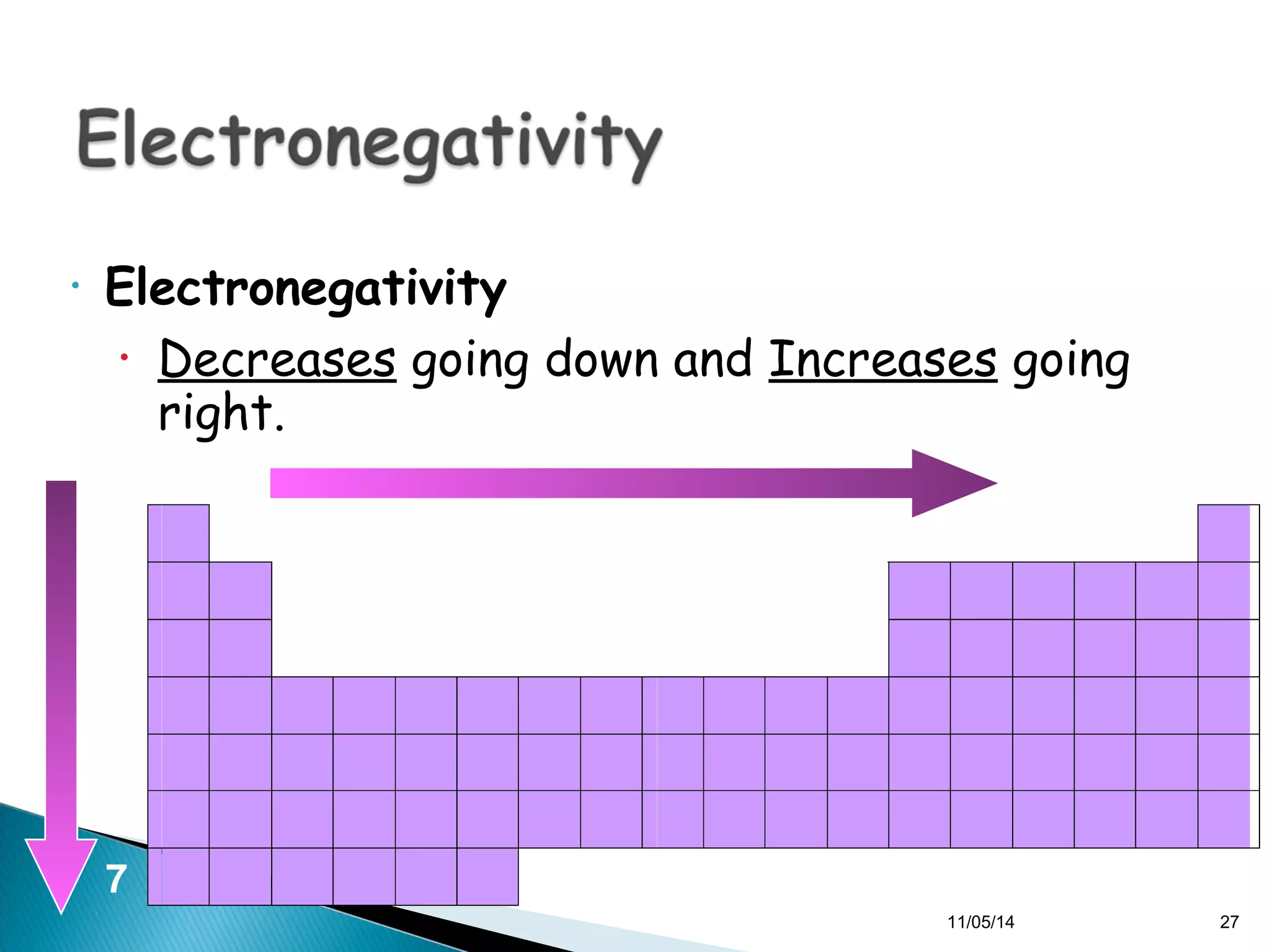
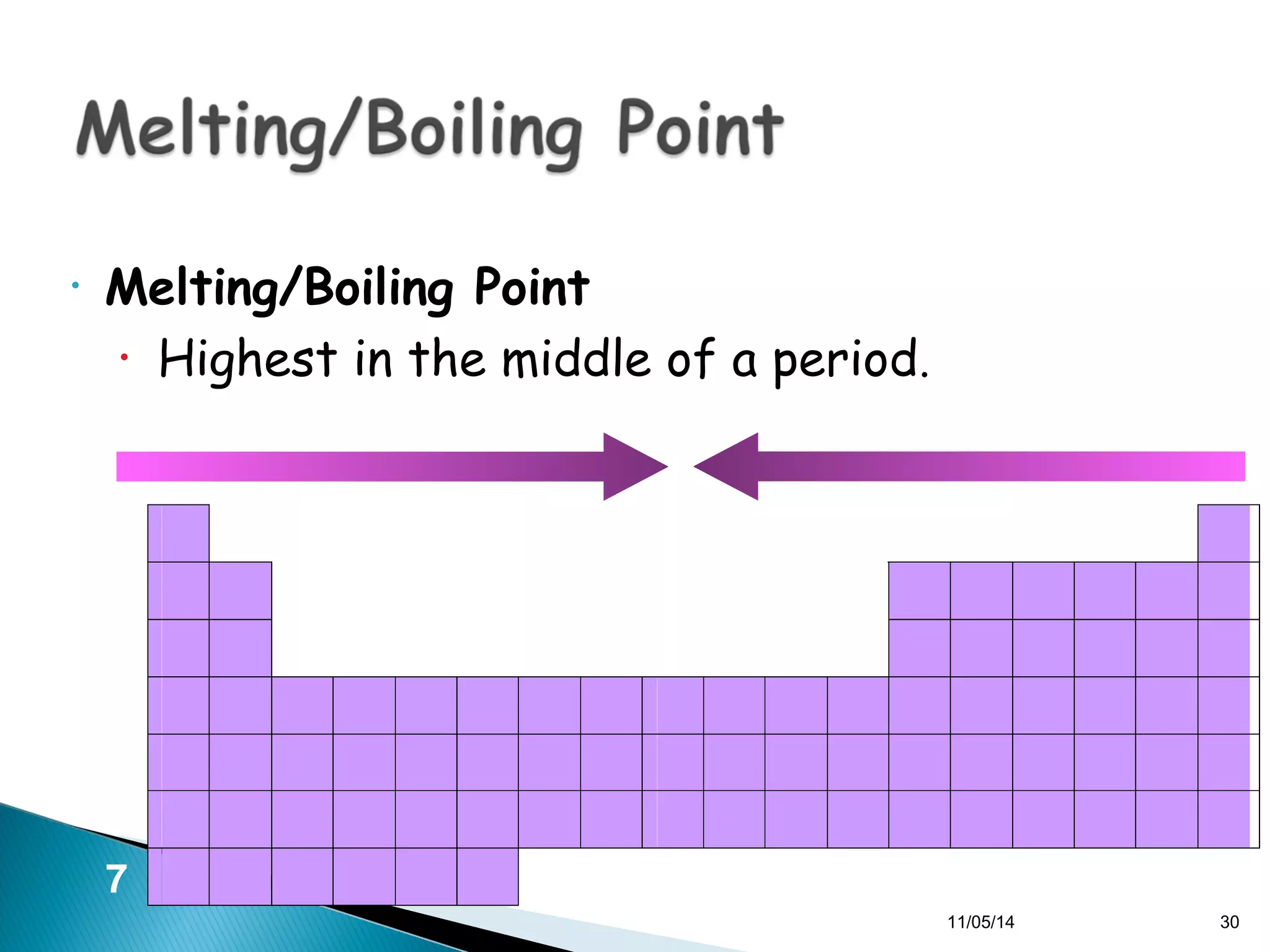
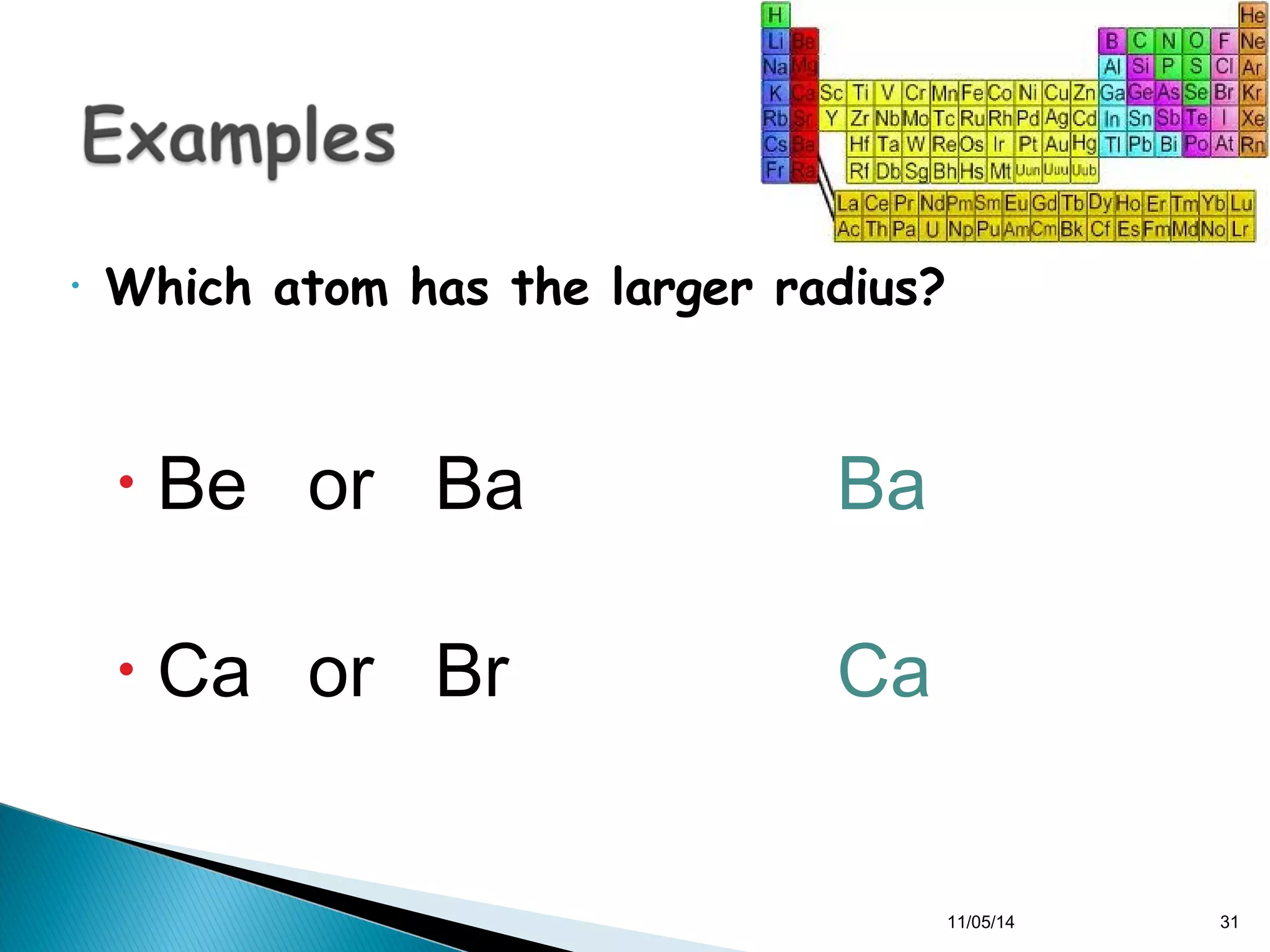
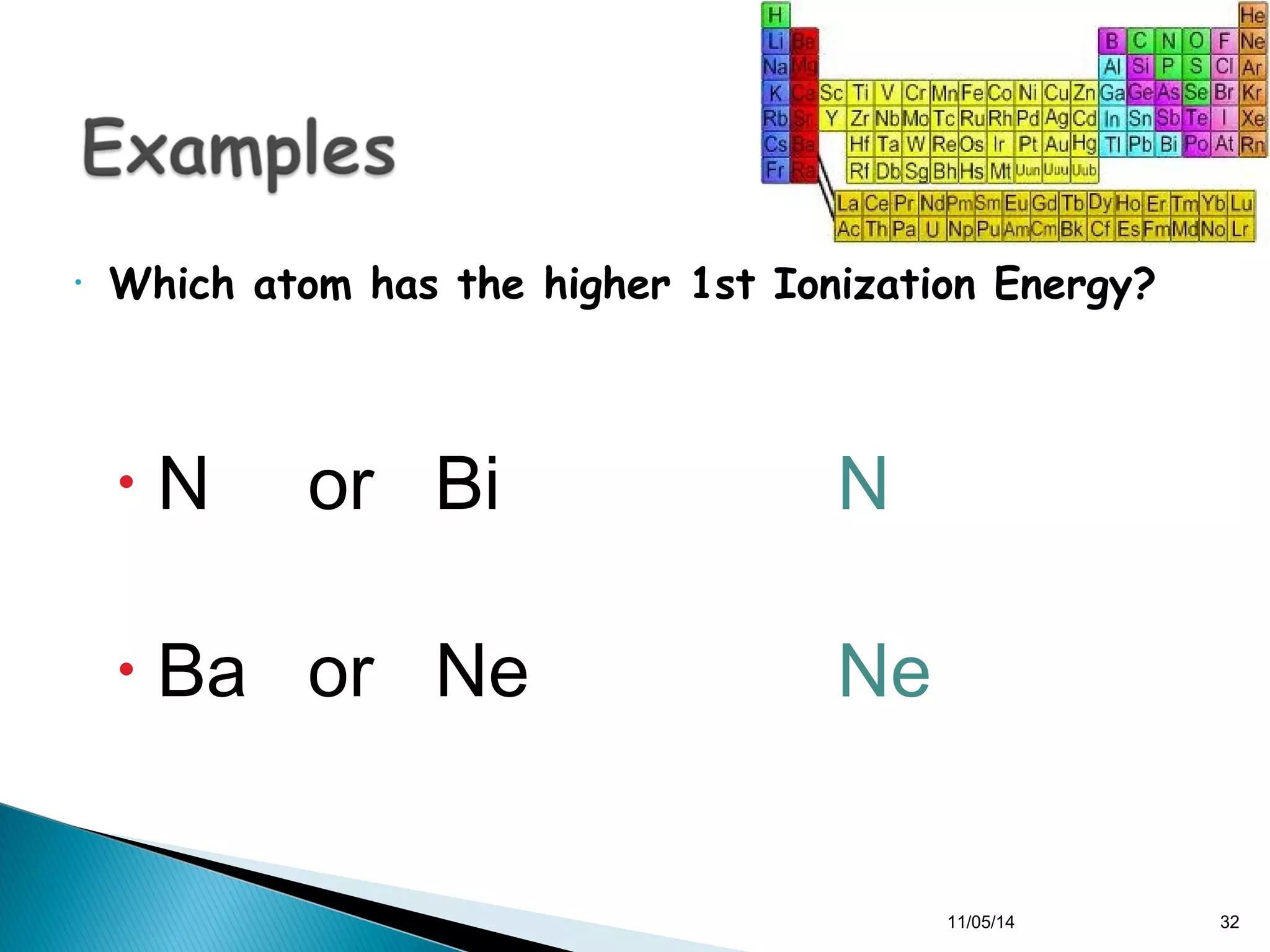
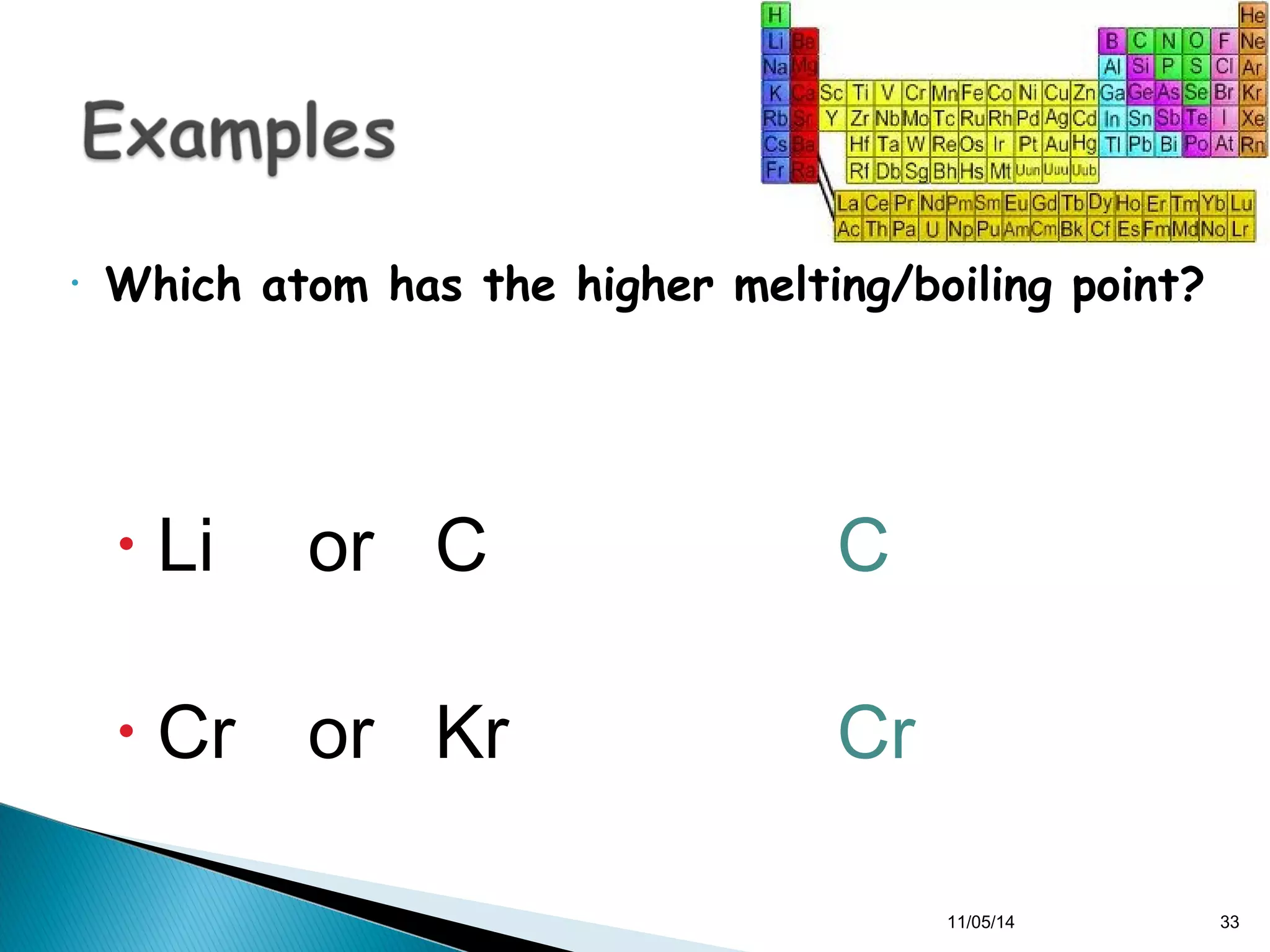
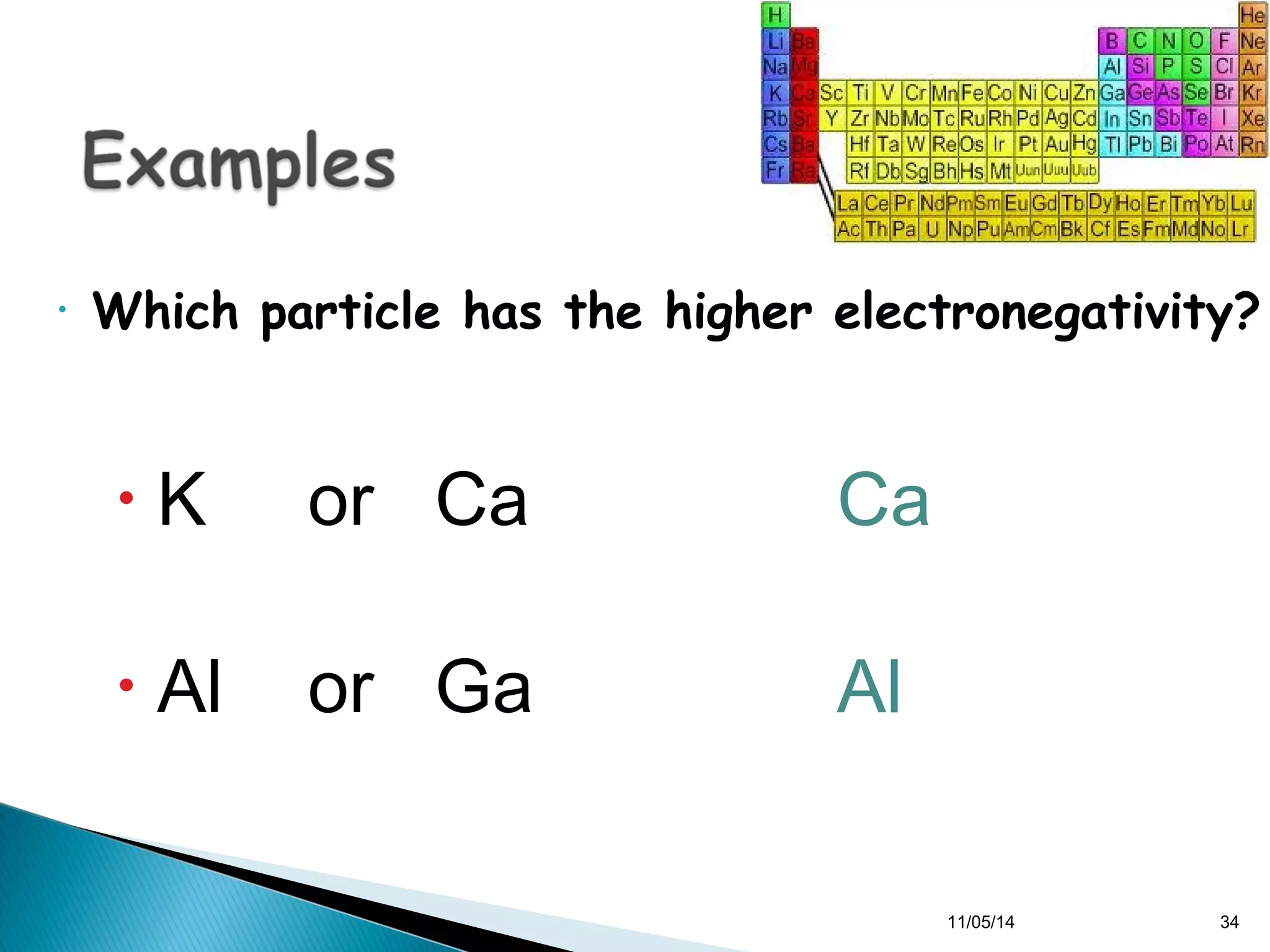
This document discusses the periodic table and periodic trends of elements. It explains how early scientists like Deboreiner, Newlands, and Mendeleev contributed to the development of the periodic table by arranging elements by atomic mass. The periodic table arranges elements by atomic number and shows trends in properties across periods and down groups, including decreasing atomic radius and ionization energy but increasing atomic radius. Metallic character decreases across periods but increases down groups.