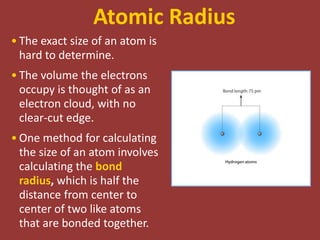
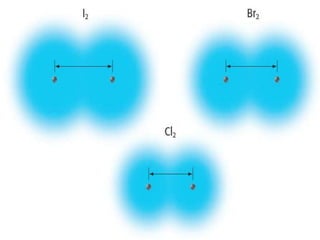
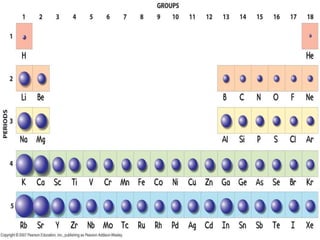
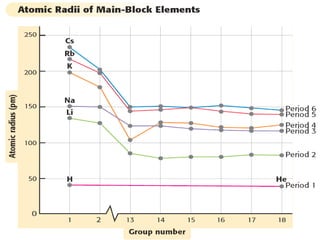
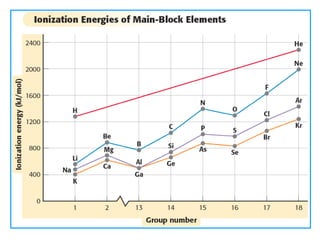
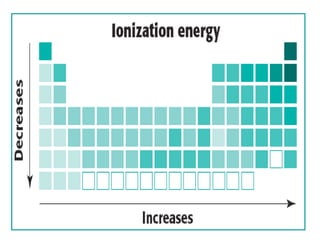
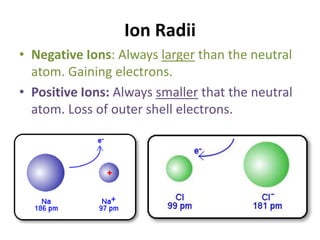
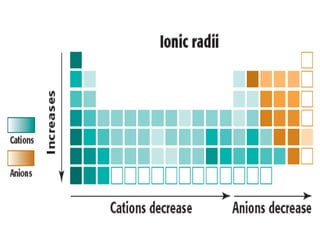
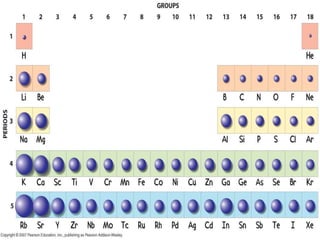
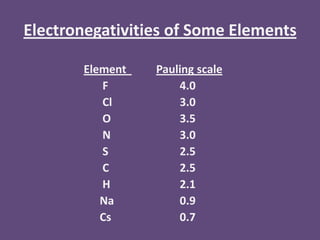
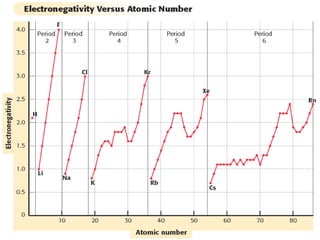
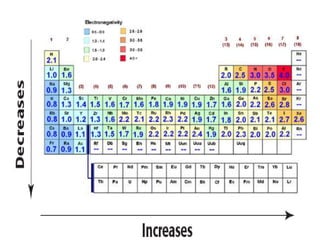
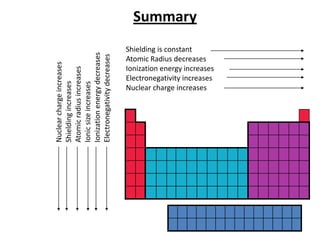
The document discusses periodic trends in atomic properties including atomic radius, ionization energy, and electronegativity. It explains that as you move down a group in the periodic table, atomic radius increases while ionization energy and electronegativity decrease due to increased electron shielding. However, as you move across a period, atomic radius decreases while ionization energy and electronegativity increase due to the stronger effective nuclear charge pulling electrons in more tightly.