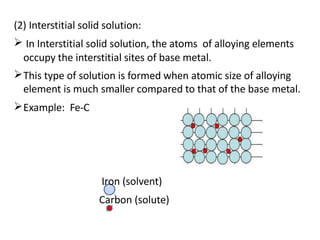
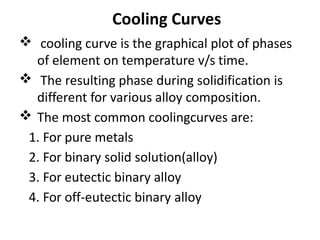
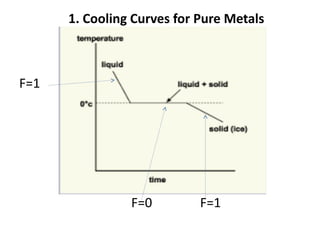
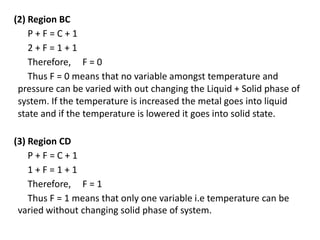
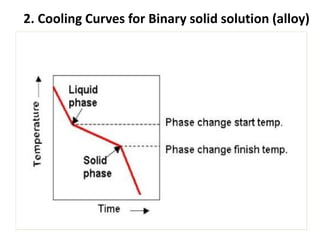
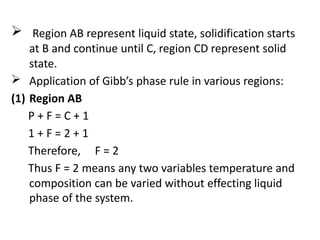
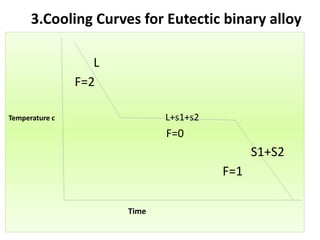
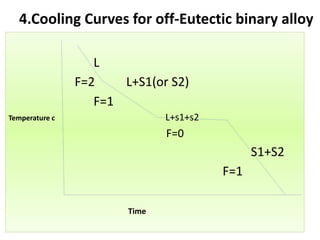
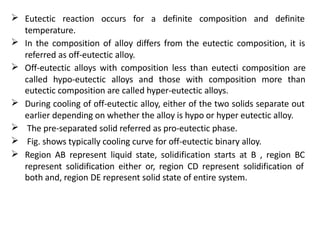
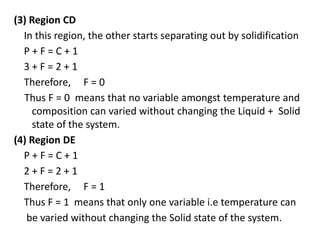
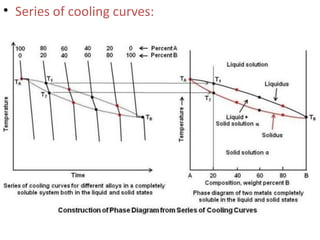
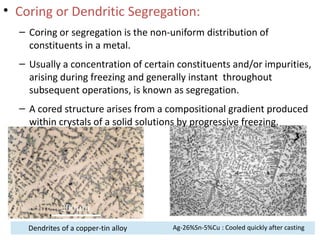
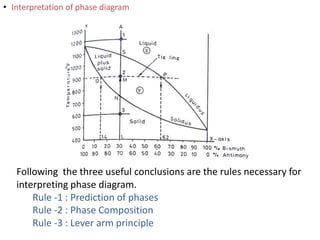
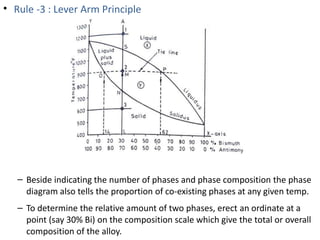
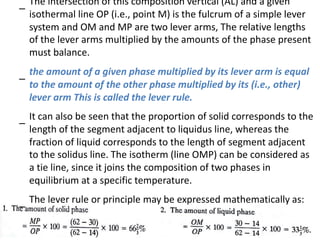
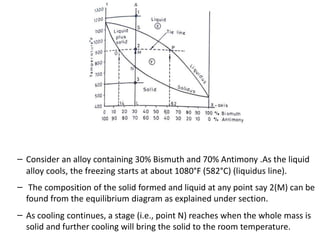
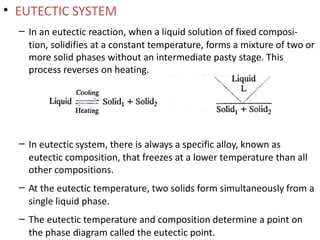
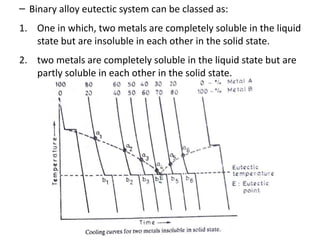
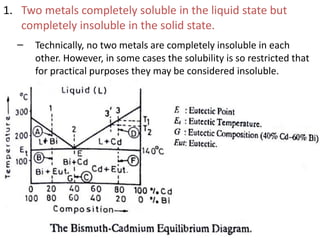
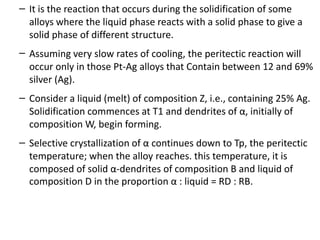
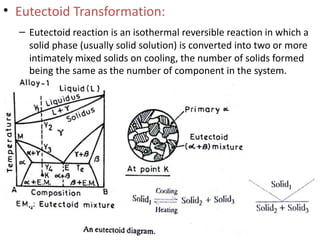
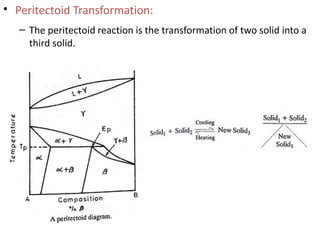
The document discusses phase diagrams and phase equilibria. It introduces key concepts like the Gibbs phase rule, cooling curves, and classification of equilibrium diagrams. The Gibbs phase rule establishes the relationship between the number of phases, components, and degrees of freedom in a system. Cooling curves show the phases present at different temperatures during solidification. The classification of equilibrium diagrams includes diagrams for pure metals, binary solutions, eutectic alloys, and off-eutectic alloys. Hume-Rothery rules govern solid solubility based on factors like atomic size and chemical affinity. Phase diagrams provide important information about phase boundaries, solubility, and temperatures of phase changes.





























![❑ Define the following terms:
[solid, liquid, gas, pure substance, compound, mixture, element, heterogeneous mixture, homogeneous mixture,
extensive properties, intensive properties, chemical properties, physical properties, density, color, texture, conductivity,
malleability, ductility, boiling point, melting point, flammability, corrosiveness, volatility, pounding, tearing, cutting,
dissolving, evaporating, fermenting, decomposing, Exothermic, endothermic, mass, density, gravity, adhesive force,
cohesive force, interface, adsorption, catalyst, dipole, physisorption, Chemisorption, hydrophilic, hydrophobic,
detergent, surfactant, surface tension, adsorbate, adsorbent, etc]
❑Respond to the following questions:
➢What is viscosity and its relationship with fluids
➢What are surface and Inter-facial tension forces and respective association with activities of a substance
material with surface area
➢How is a chemical change different from a physical change at the surface of a material
➢What is contact angle of a substance and its significant role when two materials surface are in contact
➢What is a detergent and justified reasons for its variable composition
➢What is the micelle made up of in terms of its physical form and shape
➢What are some of the practical uses of surface agent material](https://image.slidesharecdn.com/2-4-phaseequilibriumgeneric-221124230615-ecdd3a51/85/2-4-PhaseEquilibriumGeneric-pdf-65-320.jpg)