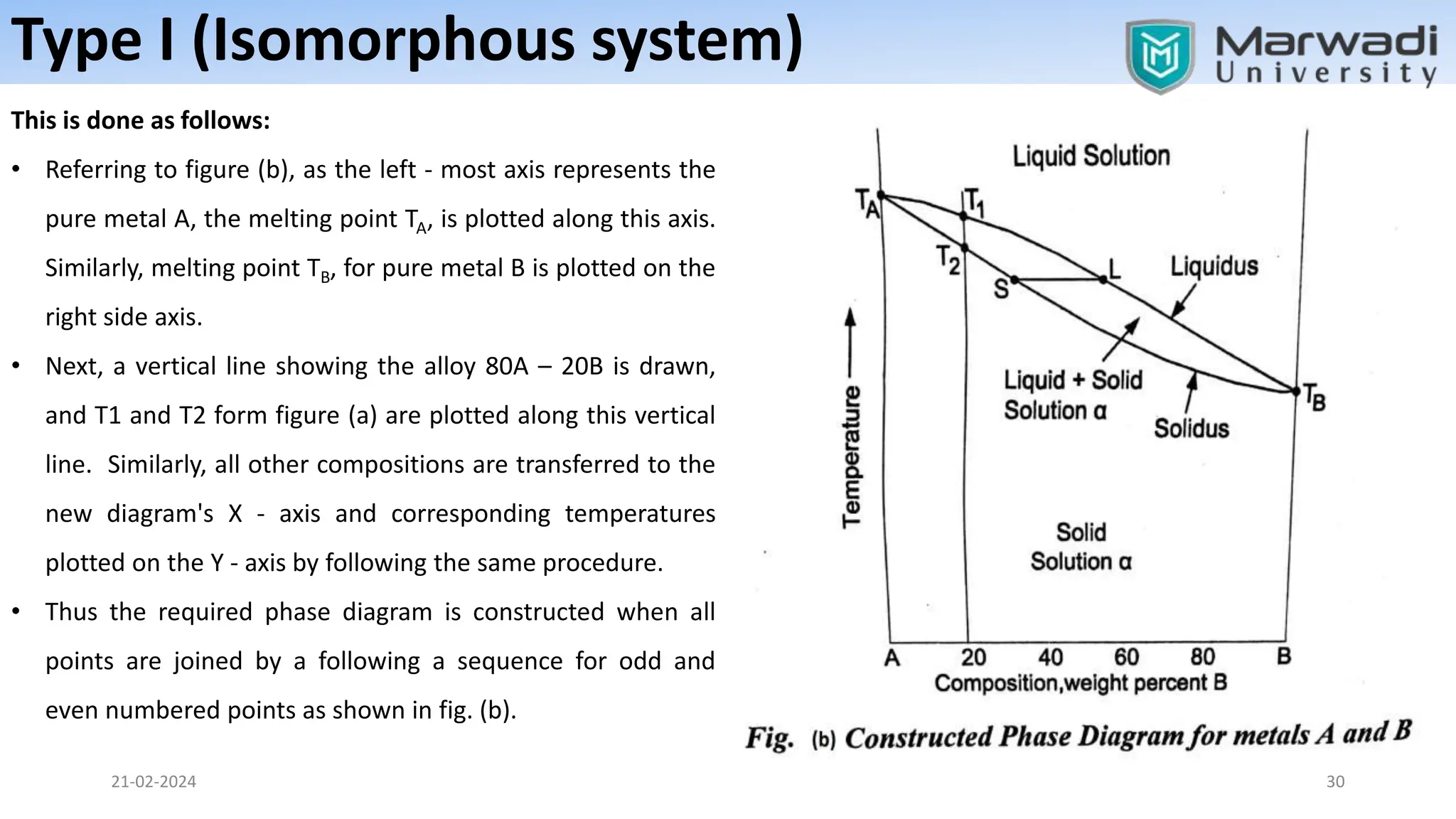
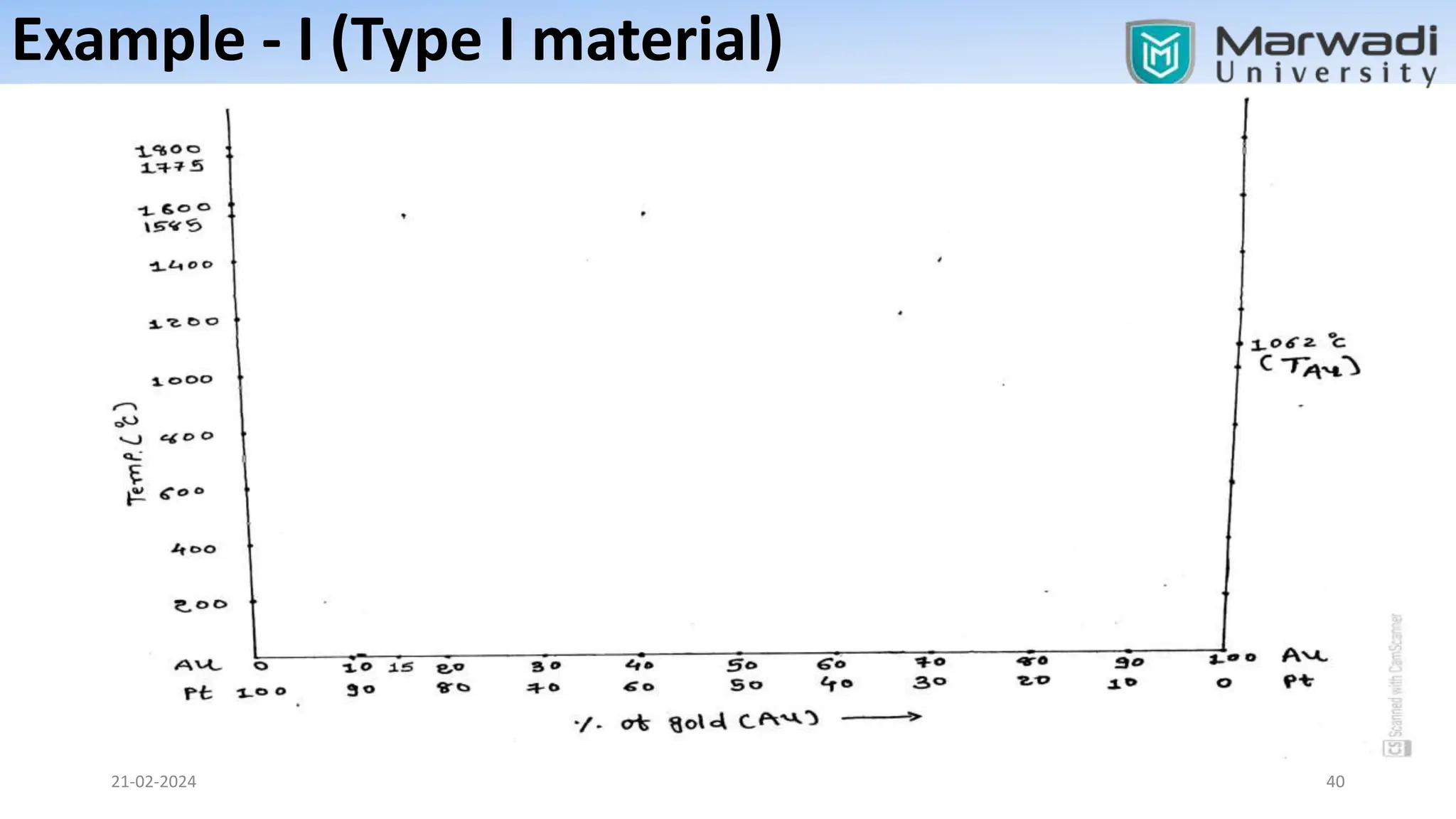
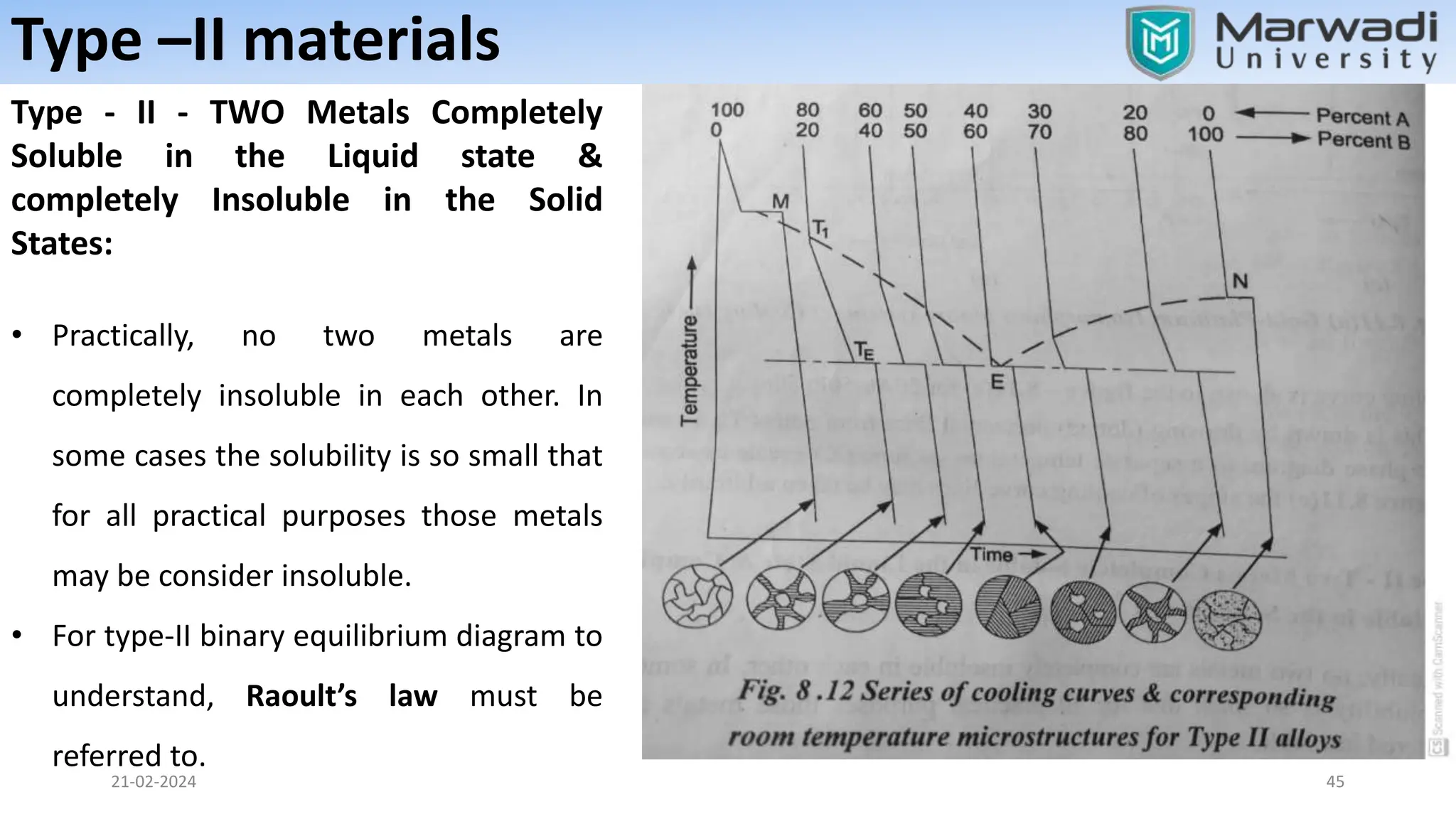
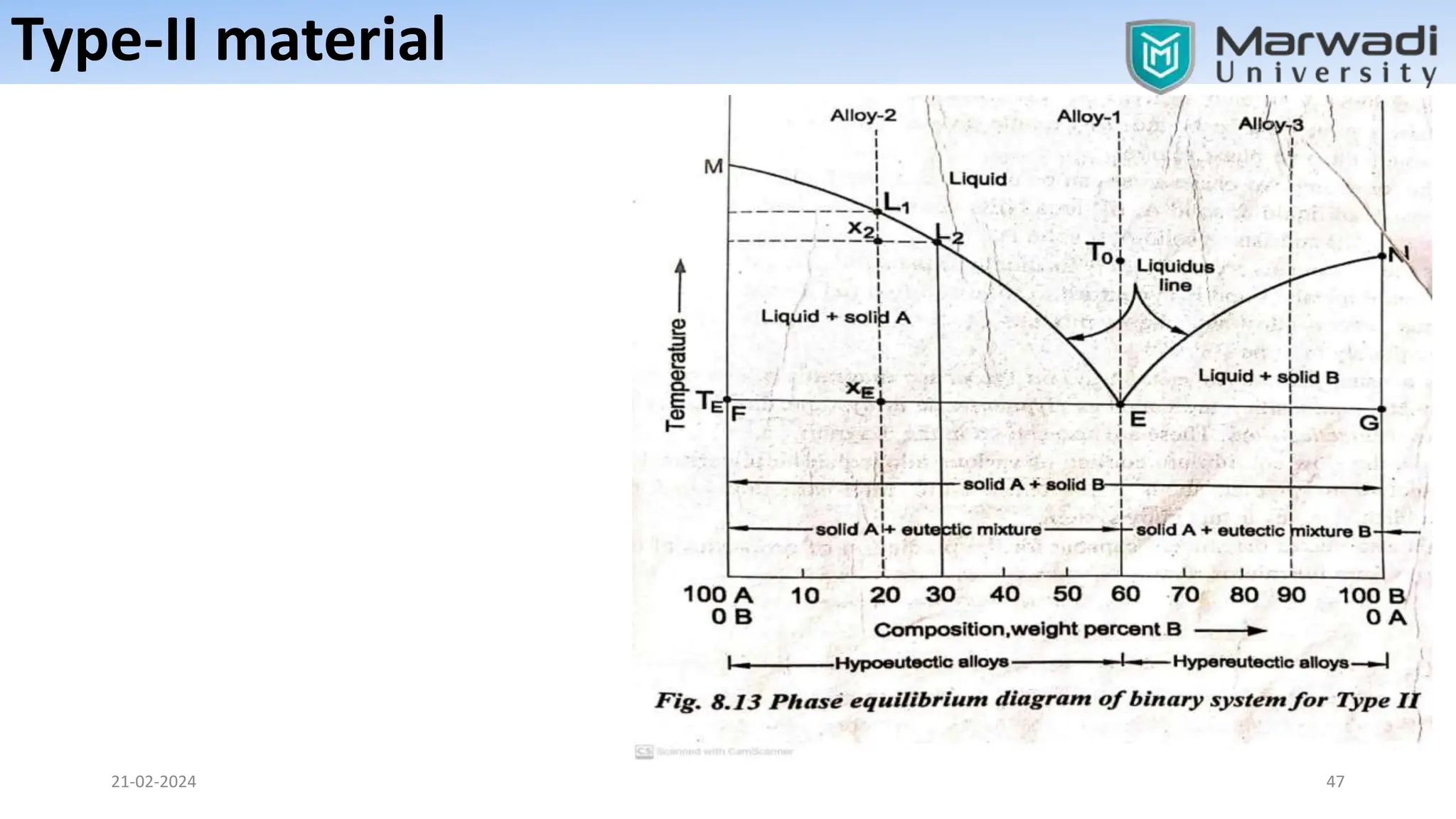
This document discusses phase diagrams and related concepts. It begins by defining key terms like phase, component, and system. It then explains the concept of phase equilibrium and how Gibbs phase rule relates the number of phases, components, and degrees of freedom in a system. The document discusses unary, binary, and ternary phase diagrams. It explains cooling curves and how they provide information about phase changes during solidification. Construction of phase diagrams using thermal analysis data from cooling curves is also covered. In summary, the document provides an overview of phase diagrams and phase equilibrium through defining concepts and discussing experimental techniques.