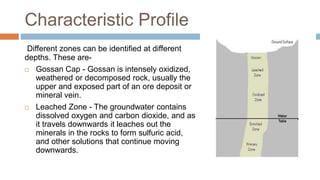
Supergene enrichment occurs when oxidizing acids dissolve metal ions from near-surface parts of ore deposits and re-deposit them at depth, resulting in higher metal concentrations. This process forms distinct zones - an oxidized cap, a leached zone, and an enriched zone below the water table where metals precipitate under reducing conditions. Common effects include rendering shallow parts of deposits barren while concentrating metals into narrow, rich zones at depth through mineral alterations and redeposition. Examples of deposits formed or enriched by this process include many copper, lead, zinc, silver, and iron deposits globally.