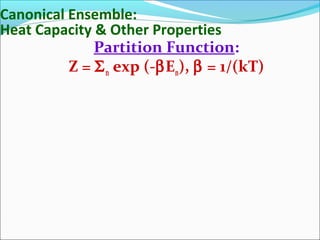
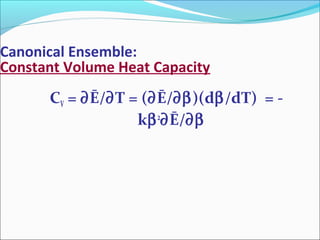
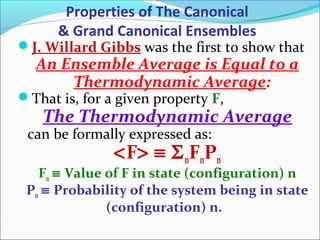
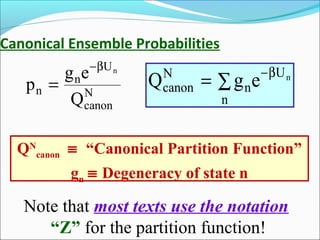
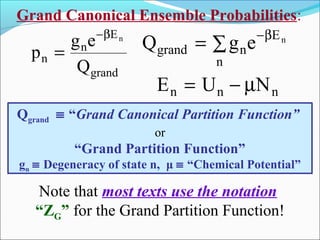
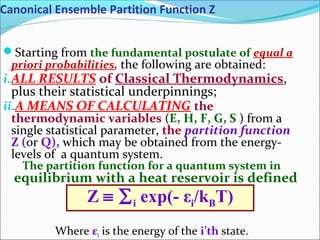
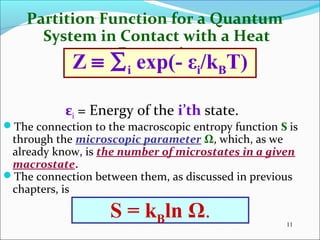
This document introduces key concepts in statistical mechanics, including the idea that macroscopic properties are thermal averages of microscopic properties. It discusses common statistical ensembles like the microcanonical ensemble (isolated systems with constant energy) and the canonical ensemble (systems in equilibrium with a heat reservoir). The canonical partition function Z relates microscopic quantum mechanics to macroscopic thermodynamics and can be used to calculate thermodynamic variables. Properties like heat capacity can be derived from fluctuations in energy calculated from the partition function.






![Relationship of Z to Macroscopic Parameters
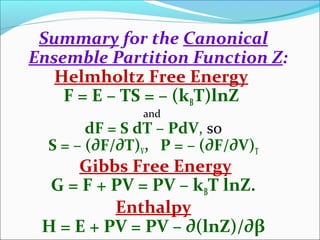
Summary for the Canonical
Ensemble Partition Function Z:
(Derivations are in the book!)
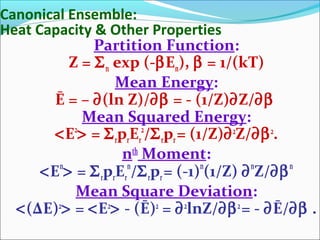
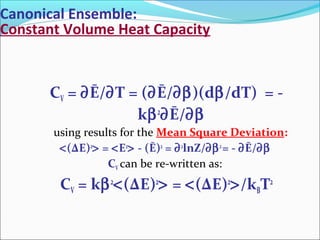
Internal Energy: Ē ≡ E = - ∂(lnZ)/∂β
<ΔE)2
> = [∂2
(lnZ)/∂β2
]
β = 1/(kBT), kB = Boltzmann’s constantt.
Entropy: S = kBβĒ + kBlnZ
An important, frequently used result!](https://image.slidesharecdn.com/yogitathakur-170410140508/85/STATISTICAL-MECHNICE-12-320.jpg)